0278
Hyperpolarized Carbon-13 Metabolic Imaging of Pediatric Patients with Brain Tumors: Initial Experience1Radiology, Chonnam National University College of Medicine, Gwanju, Republic of Korea, 2Radiology and Biomedical Imaging, University of California San Francisco, San Francisco, CA, United States, 3Pediatric Hematology-Oncology, University of California San Francisco, San Francisco, CA, United States, 4Neurology, Neurosurgery and Pediatrics, University of California San Francisco, San Francisco, CA, United States
Synopsis
This study applied hyperpolarized 13C metabolic imaging in pediatric populations for the first time to our knowledge. Dynamic 13C data were acquired following injection of hyperpolarized [1-13C]pyruvate in the first 3 pediatric patients with brain tumors as part of an ongoing trial (
Introduction
Recent
clinical studies using hyperpolarized (HP) 13C metabolic imaging
showed the feasibility of this technology for evaluating real-time metabolism
in adults1-3. The purpose of this clinical trial is to assess the
safety and feasibility of HP 13C metabolic imaging with [1-13C]pyruvate
for evaluating real-time metabolism in pediatric patients with brain tumor. To
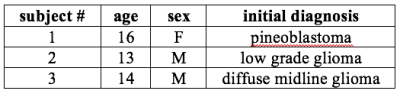
date, dynamic 13C data were obtained from 3 pediatric patients with
a prior diagnosis of brain tumor using 13C coils and pulse sequences
that were previously designed for brain application3.Methods
All experiments were performed under a UCSF IRB approved protocol, using a whole-body GE 3T MR scanner with a clamshell volumetric 13C transmit coil and bi-lateral phased array receive coils that each had 4 elements. A mixture of 1.432g of GMP [1-13C]pyruvic acid and 28 mg of trityl radical was polarized using a SpinLabTM (General Electric, Niskayuna, NY) system. After approximately 2.5 hours of polarization and the subsequent dissolution, the pyruvate solution underwent QC testing to determine the pH, temperature, polarization level and sterilization filter integrity, as well as the concentrations of pyruvate and the residual electron paramagnetic agent (EPA) used in the preparation. Following pharmacist approval, the agent was quickly transferred to the scanner room and 0.34mL/kg pyruvate solution (~250mM; dose level 1 (=80% of adult recommended dose) followed by 20mL saline flush was administered at a rate of 5 mL/s. Dynamic 2D 13C spectroscopic imaging data were acquired starting at 5s from the end of pyruvate+saline injection from a 20 mm thick slice centered over suspected tumor using a constant 10° flip angle (24 total time points) with 10 phase encodes in the RL direction and a symmetric echo-planar readout in the AP direction (TE/TR=6.1/130ms, 3s temporal resolution, 20x20mm nominal in-plane resolution)4. The 13C data were processed and analyzed using in-house software5,6.Results and Discussion
No adverse effects were observed: All patients on dose level 1 tolerated the pyruvate injection well and no adverse effects were observed (n=3). Within the ongoing trial, subsequent patients will receive the adult recommended dose (0.43mL/kg).
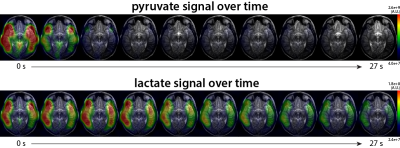
Hyperpolarized 13C metabolic imaging was able to detect metabolic processes in pediatric human brain: Figure 1 shows an example of HP 13C data from patient #1. The dynamic pyruvate and lactate signal demonstrated the spatial and temporal evolution of 13C-labeled pyruvate and lactate resonances throughout the brain. The pyruvate appeared in multiple regions, with the maximum pyruvate near the superficial middle cerebral vein appearing immediately after the start of data acquisition. The pyruvate signal decreased rapidly from the maximum peak. Maximum lactate signal was observed approximately 6s from the start of data acquisition, and appeared throughout normal-appearing brain.
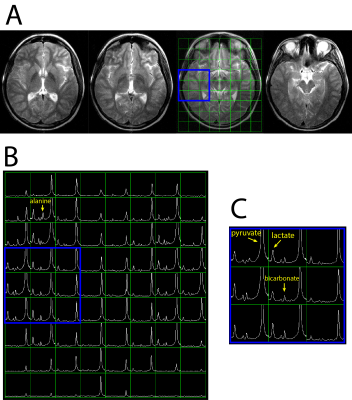
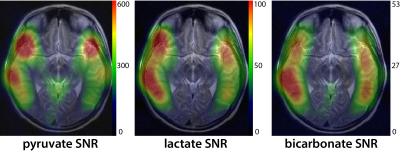
Hyperpolarized 13C metabolic imaging was able to detect bicarbonate as well as pyruvate and lactate with high SNR: Figure 2 shows an example of integrated HP 13C spectra from patient #1. T2 fast spin-echo (FSE) images (Fig 2A) with grid overlays correspond to integrated 13C spectra in Figure 2B. The spectra over the whole brain (Fig 2B) as well as the zoom-in region (Fig 2C) demonstrated excellent detection of brain 13C metabolites. The coil setup with the clamshell transmit and the 8-channel bilateral receive arrays provided the acquisition of 13C signals across the majority of the brain, but with significantly lower SNR in central regions. The low SNR in the center of the brain was worsened by the reduced signal in the ventricles. Figure 3 shows maps of integrated pyruvate, lactate and bicarbonate SNR. The maximum SNR of pyruvate, lactate and bicarbonate were 791, 105 and 54, respectively.
Conclusions
This study focused on the novel application of hyperpolarized 13C metabolic imaging to pediatric brain tumor patients. The preliminary results from this study have demonstrated initial safety and feasibility of using HP [1-13C]pyruvate for assessing in vivo metabolism in pediatric patients with brain tumors and its potential for future applications.Acknowledgements
The support for the research studies came from NIH grants R01EB013427, P41EB013598 and R21CA170148, Kure It Grant for Underfunded Cancer Research, The Pediatric Brain Tumor Foundation, Discovery Grant from American Brain Tumor Association, and the National Research Foundation (NRF) of Korea grant funded by Ministry of Science and ICT (No.2017R1C1B5018396).References
1. Nelson SJ, Kurhanewicz J, Vigneron DB, Larson PE, Harzstark AL, Ferrone M, et al. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-(1)(3)C]pyruvate. Science translational medicine. 2013;5(198):198ra08.
2. Cunningham CH, Lau JY, Chen AP, Geraghty BJ, Perks WJ, Roifman I, et al. Hyperpolarized 13C Metabolic MRI of the Human Heart: Initial Experience. Circulation research. 2016;119(11):1177-82.
3. Park I, Larson P, Gordon J, Carvajal L, Chen H, VanCriekinge M, et al. Dynamic Hyperpolarized 13C Metabolic Imaging of Patients with Brain Tumors. Proceedings of ISMRM 25th meeting. P0555.
4. Larson PE, Hu S, Lustig M, Kerr AB, Nelson SJ, Kurhanewicz J, Pauly JM, Vigneron DB. Fast dynamic 3D MR spectroscopic imaging with compressed sensing and multiband excitation pulses for hyperpolarized 13C studies. Magn Reson Med. 2011 Mar;65(3):610-9.
5. Nelson SJ Analysis of volume MRI and MR spectroscopic imaging data for the evaluation of patients with brain tumors. Magn Reson Med 2001;46:228-39.
6. Crane JC, Olson MP, Nelson SJ. SIVIC: Open-Source, Standards-Based Software for DICOM MR Spectroscopy Workflows. Int J Biomed Imaging. 2013;2013:169526.
Figures