0275
Overestimation of cardiac lactate production due to liver metabolism of hyperpolarized [1-13C] pyruvate1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
Spectroscopy is widely used in hyperpolarized metabolic experiments due to its simplicity and robustness. In this work, it is shown that with spectroscopic acquisition in rat hearts, the cardiac lactate production is overestimated due to liver metabolism of [1-13] pyruvate. It is demonstrated that this overestimation can be addressed using spatially resolved data.
Introduction
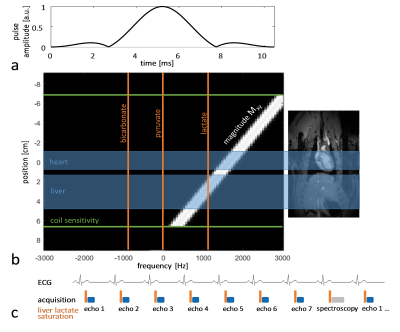
Dissolution Dynamic Nuclear Polarization (DNP) is a promising tool to study cardiac metabolism1. Spectroscopic acquisitions have been used in many studies due their simple implementation and robustness against frequency offsets and B0 inhomogeneity. Localization is typically performed with either slice selection or surface coil positioning. The lack of spatial resolution in spectroscopy makes it however impossible to separate signal contributions from different compartments (e.g. blood pool and myocardium in the heart). The liver produces lactate from hyperpolarized pyruvate2 and this lactate pool is partly released into the blood stream, flows to other organs and contributes to the detected hyperpolarized lactate in these other organs. In this work, it is shown that lactate produced by the liver contaminates the signal detected in the heart. Selective lactate saturation in the liver is used to quantify this contamination. Spectroscopic acquisition is compared to metabolic imaging using a dedicated sequence that allows interleaved acquisition during the same experiment.Methods
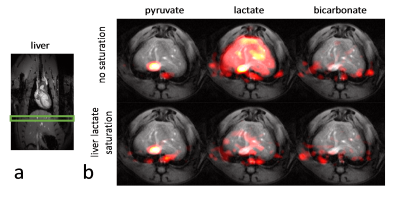
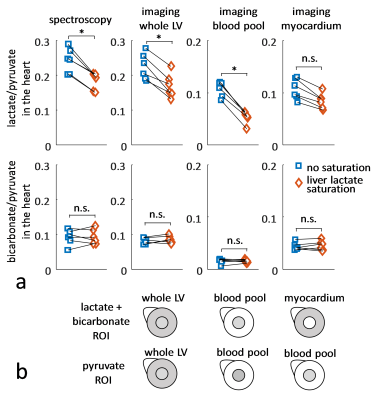
Experiments were carried out on a Bruker BioSpec 9.4T system. All animal experiments were performed in adherence to the Swiss Animal Protection law. A RF saturation pulse (length 10.5ms) was implemented to allow for selective lactate saturation in the liver (Figure 1). The pulse was tested in an imaging experiment with an axial slice through the liver (Figure 2). Imaging was performed with a multi-echo single-shot EPI acquisition3 at 1.25x1.25x3.5 mm3 resolution. Six healthy Sprague Dawley rats were then used to compare cardiac slice-selective spectroscopy with cardiac metabolic imaging. Each rat received two injections of hyperpolarized [1-13C] pyruvate separated by 20 minutes. Hyperpolarization was performed with a home-built DNP polarizer.4 One scan was performed with liver lactate saturation and the other without. The order of the two scans was randomized. Interleaved slice-selective spectroscopy and imaging was implemented as shown in Figure 1c with a TR ≈ 1.2 sec. Spectroscopic data were quantified using Amares in jMRUI. Imaging data were segmented with three regions of interest: 1) whole left ventricle (LV), 2) LV blood pool and 3) LV myocardium. Both spectroscopic and imaging data were integrated along time to obtain the area-under-the-curve (AUC)5 of signal-time curves and metabolite ratios were calculated. Statistical significance of the difference of metabolite ratios between scans with and without liver lactate saturation was determined using repeated measures ANOVA.Results
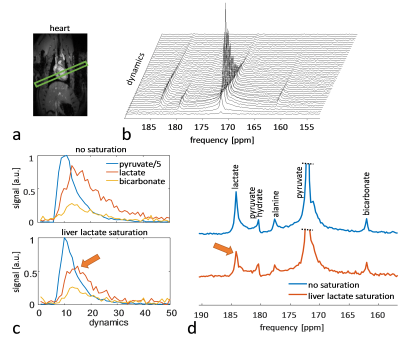
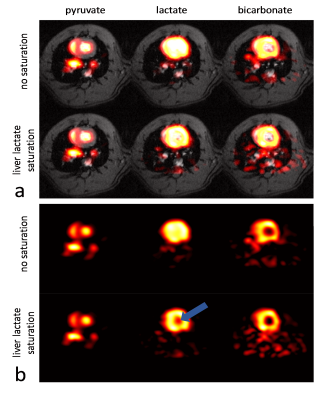
The imaging data of the liver (Figure 2) show that without saturation, the liver produces relatively high amounts of lactate, which can be suppressed using the saturation pulse. Figure 3 shows example spectra acquired from the heart with and without liver lactate saturation. Figure 4 shows example imaging data acquired from the heart with and without liver lactate saturation. Figure 5 compares the metabolite ratios obtained from spectroscopic and imaging data for the cases with and without liver lactate saturation, respectively. When using liver lactate saturation, there is a significant reduction of lactate in cardiac spectroscopy, as well as of the whole left-ventricular (LV) lactate in imaging and the LV blood pool lactate in imaging, however not of the LV myocardial lactate (Figure 5).Discussion
24 ± 5% of the lactate signal in cardiac slice-selective spectroscopy was found to originate from lactate that was produced by the liver and flowed to the heart. Therefore, changes in liver metabolism will compromise cardiac spectroscopic data and might be wrongly attributed to changes in cardiac metabolism. Cases that have been studied with hyperpolarized pyruvate include fasted and fed states of the subject2, metabolic diseases such as diabetes6 and systemic administration of drugs7, all of which can be expected to affect the metabolism of multiple organs simultaneously. It is therefore crucial to separate the signal originating from different organs in order to establish accurate metabolic data of the heart. Since imaging allows distinguishing between spatial compartments, it should be preferred over spectroscopy. Liver lactate saturation was not found to affect the myocardial lactate signal in imaging data significantly, however there might still be some contamination of liver lactate into the myocardium due to signal spilling from the blood pool as result of the spatial point-spread function. Therefore, saturation of unwanted signal should be considered for metabolic studies.Conclusion
Liver metabolism leads to statistically significant overestimation of lactate detected in slice-selective spectroscopy experiments in the heart. Imaging allows separating the blood pool from the myocardium and should therefore be preferred. Saturation of unwanted signal should be considered for future metabolic studies.Acknowledgements
The authors acknowledge funding from the Swiss National Science Foundation, grant 320030_153014.References
1. Schroeder MA, Clarke K, Neubauer S, Tyler DJ. Hyperpolarized Magnetic Resonance A Novel Technique for the In Vivo Assessment of Cardiovascular Disease. Circulation 2011;124:1580–1594.
2. Hu S, Chen AP, Zierhut ML, Bok R, Yen Y-F, Schroeder MA, Hurd RE, Nelson SJ, Kurhanewicz J, Vigneron DB. In Vivo Carbon-13 Dynamic MRS and MRSI of Normal and Fasted Rat Liver with Hyperpolarized 13C-Pyruvate. Mol Imaging Biol 2009;11:399.
3. Sigfridsson A, Weiss K, Wissmann L, Busch J, Krajewski M, Batel M, Batsios G, Ernst M, Kozerke S. Hybrid multiband excitation multiecho acquisition for hyperpolarized 13C spectroscopic imaging. Magn Reson Med 2015;73:1713–1717.
4. Krajewski M, Wespi P, Busch J, Wissmann L, Kwiatkowski G, Steinhauser J, Batel M, Ernst M, Kozerke S. A multisample dissolution dynamic nuclear polarization system for serial injections in small animals. Magn Reson Med 2017;77:904–910.
5. Hill DK, Orton MR, Mariotti E, et al. Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PLoS ONE 2013;8:e71996.
6. Le Page LM, Ball DR, Ball V, Dodd MS, Miller JJ, Heather LC, Tyler DJ. Simultaneous in vivo assessment of cardiac and hepatic metabolism in the diabetic rat using hyperpolarized MRS. NMR Biomed 2016;29:1759–1767.
7. Lewis AJM, Miller JJJ, McCallum C, Rider OJ, Neubauer S, Heather LC, Tyler DJ. Assessment of Metformin-Induced Changes in Cardiac and Hepatic Redox State Using Hyperpolarized[1-13C]Pyruvate. Diabetes 2016;65:3544–3551.
Figures