0274
Hyperpolarized sodium [1-13C]glycerate as a probe for assessing glycolysis in vivo1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Electrical and Computer Engineering, University of Texas at Dallas, Richardson, TX, United States, 4Chemistry and Biochemistry, San Francisco State University, San Francisco, CA, United States, 5Stanford University, Stanford, CA, United States, 6Chemistry and Biochemistry, California State University, Fullerton, Fullerton, CA, United States
Synopsis
We describe the synthesis, development and in vivo application of sodium [1-13C]glycerate as a novel probe for evaluating glycolysis using hyperpolarized 13C MRS. [13C]glycerate displayed a high level of polarization and long spin-lattice relaxation time. In vivo spectroscopic studies with hyperpolarized [13C]glycerate in rat liver furnished metabolic products, pyruvate and lactate, originating from glycolysis. The levels of production and relative intensities of these metabolites were directly correlated with the induced glycolytic state (fasted versus fed). This work establishes hyperpolarized [13C]glycerate as a novel agent for clinically relevant 13C MRS studies of energy metabolism and further provides opportunities for evaluating intracellular redox states in biochemical investigations.
Background
Glycolysis is a highly conserved pathway that furnishes ATP and NADH via the conversion of glucose to pyruvate. Tissue specific variations of glycolysis activity are found in order to balance the physiological needs of distinct cellular environments, and the pathway can be operative under both aerobic and anaerobic conditions1,2. The Warburg effect, for example, is a distinctive observation in cancer biology in which tumor cells exhibit higher levels of glycolysis even under aerobic conditions. In addition, pyruvate kinases, which catalyze the final rate-limiting step of converting phosphoenolpyruvate to pyruvate, display differential expression patterns in cancer cells, and the isozyme PKM2 has emerged as a novel target for cancer therapeutics3. Alterations in glycolytic metabolism have further been correlated with oxidative stress and changes in intracellular redox state. Glycerate-based substrates were an attractive scaffold for our initial investigations, as glycerate has the potential to be metabolized via glycolysis. In addition, the C1-carbonyl carbon of glycerate (Glyc) could potentially be 13C-labeled. Importantly, this labeling strategy would: (1) obviate the need for 1H-decoupling, (2) provide suitable T1 for the substrate and resulting C1-labeled metabolites, and (3) allow for longer experimentation times during hyperpolarized 13C metabolic studies.Methods
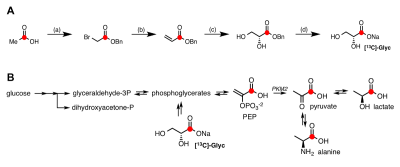
The synthetic procedure of [13C]-Glyc is shown in Fig.1A. The samples to be polarized consisted of a mixture of [13C]-Glyc in 3.0 M glycerol/water (3/2, v/v) solution containing 15 mM trityl radical OX063 (Oxford Instruments Molecular Biotools). The samples were polarized using either a SPINlab (GE Healthcare) or HyperSense system (Oxford Instruments Molecular Biotools). Perfused liver studies were carried out in two groups of mice (fast vs. fed) using an 89-mm vertical 9.4 T NMR spectrometer and the HyperSense as described in Ref.4. For in vivo study, healthy male Wistar rats (fed vs. fasted, n = 3/group) were injected with the hyperpolarized solution (1mmol/kg) through a tail vein catheter. All in vivo experiments were performed on a clinical 3T MR scanner (GE Healthcare, Waukesha, WI), using a custom-built 13C transmit/receive surface coil placed over the liver with rat supine. A non-selective pulse-and-acquire sequence with a small flip angle excitation (spectral width=5kHz, 2,048 points) was used to acquire 13C spectra from the liver.
Results and Discussion
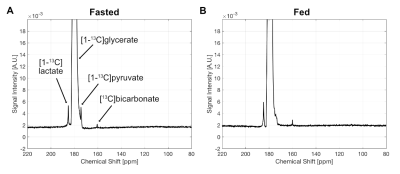
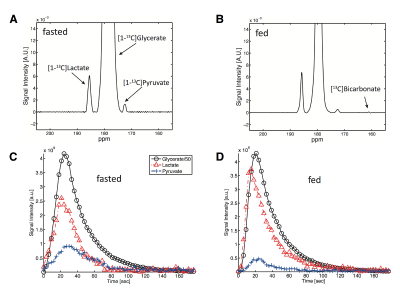
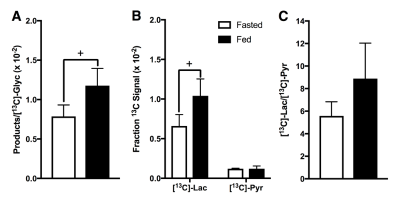
The synthetic route provided [13C]-Glyc in a 56% yield over four steps. Upon dissolution, 19.7% ± 0.9% (n=3) liquid-state polarization was detected at the scanner (~35 s after dissolution, SPINlab). The T1 of the 13C-labeled carbonyl was determined to be 59.9 ± 3.0 s at 3T in the hyperpolarized solution, which importantly represented a significant increase in relaxation time relative to previous hyperpolarized agents, [2H,13C]-glucose5 and [13C]-dihydroxyacetone4. In the fed state, glucose is metabolized via glycolysis to generate pyruvate, which further generates ATP through the TCA cycle and oxidative phosphorylation in the mitochondria. However, during prolonged fasting, glycolysis activity is diminished, and hepatocytes synthesize glucose from precursors (e.g., lactate, pyruvate, glycerol and amino acids) via gluconeogenesis. These opposing physiological states further alter the redox environment in the liver, typically resulting in a more reduced state under fasted conditions. Both perfused liver (Fig.2) and in vivo (Fig.3) studies showed similar results. Figure 4 summarizes the metabolic differences obtained from the fasted versus fed groups in 13C MRS experiments employing hyperpolarized [13C]-Glyc. The contribution of the metabolites was determined by examining the area under the curve for the time courses. The total signal from downstream products provided an estimation of flux in both states. Glycolytic activity was reduced by 34% in the fasted versus fed groups (P<0.05). These results with hyperpolarized [13C]-Glyc were consistent with the gluconeogenic state, where carbon precursors are consumed for de novo glucose synthesis. When the individual metabolite levels were examined as a fraction of total 13C signal (Fig.4B), [13C]-Lac displayed a significant decrease in the fasted versus fed groups (P < 0.05). Previous studies of liver metabolism have also found similar changes in lactate pool sizes6. In our in vivo experiments with hyperpolarized [13C]-Glyc, [13C]-Lac/[13C]-Pyr ratios were determined to 8.89 ± 3.15 for the fed group. Under normal physiological conditions, the cytosolic lactate/pyruvate ratio is maintained at ~10/1, correlating strongly with the hyperpolarized [13C]-Glyc analysisConclusion
In summary, we have described a novel spectroscopic strategy to probe glycolysis using hyperpolarized [1-13C]glycerate. The 13C-labeled substrate was synthesized in high yield from inexpensive, commercially available starting materials. Upon formulation of [1-13C]glycerate for DNP studies, the substrate displayed high polarization levels with a long spin-lattice relaxation time, obviating the clinical challenges associated with related agents. Importantly, hyperpolarized [1-13C]glycerate successfully targeted the glycolytic pathway in vivo, and metabolic products, [13C]pyruvate and [13C]lactate, were observed. Metabolite levels were correlated with the glycolytic state in rat liver, which establish hyperpolarized [1-13C]glycerate as a promising diagnostic probe for analyzing glycolysis.Acknowledgements
Funding support: National Institutes of Health of the United States (SC2 CA200520, R01 EB019018, R01 CA176836, S10 OD012283, P41 EB015891, P41 EB015908, S10 OD018468); The Mobility Foundation; The Texas Institute of Brain Injury and Repair;References
1. Wiśniewski, J. R.; Gizak, A.; Rakus, D. J. Proteome Res. 2015, 14, 3263–3273.
2. Lunt, S. Y.; Vander Heiden, M. G. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464.
3. Vander Heiden, M. G.; Christofk, H. R.; Schuman, E.; Subtelny, A. O.; Sharfi, H.; Harlow, E. E.; Xian, J.; Cantley, L. C. Biochem. Pharmacol. 2010, 79, 1118–1124
4. Moreno, K. X.; Satapati, S.; DeBerardinis, R. J.; Burgess, S. C.; Malloy, C. R.; Merritt, M. E. J. Biol. Chem. 2014, 289, 35859–35867.
5. Rodrigues, T. B.; Serrao, E. M.; Kennedy, B. W. C.; Mikko, D. H.; Kettunen, I.; Brindle, K. M. Nat. Med. 2014, 20, 93–97
6. Veech, R. L.; Eggleston, L. V.; Krebs, H. A. Biochem. J. 1969, 115, 609–619.
Figures