0273
An in vivo metabolic imaging study of myopathy in transgenic mice using C-13 hyperpolarized pyruvate generated by ParaHydrogen1Molecular Biotechnology and Health Sciences, University of Torino, Torino, Italy
Synopsis
Hyperpolarized [1-13C]pyruvate has been widely exploited for the in vivo investigation of metabolic processes under normal and diseased conditions. The possibility to obtain it using the cost effective and fast PHIP (ParaHydrogen Induced Polarization) method would allow a widespread application of this powerful diagnostic tool to pre-clinical research and would pave the way to future clinical translation. Here we show the first in vivo studies carried out on genetically modified mice using [1-13C]pyruvate obtained by means of the PHIP-SAH (PHIP-Side Arm Hydrogenation) method. The results obtained from PHIP-SAH hyperpolarized pyruvate are consistent with the pathologic state of the heart tissue.
Introduction
[1-13C]-pyruvate is at a central crossroad of cellular metabolism as it leads to energy production as well as to the formation of lactate and alanine . It is the hyperpolarized (HP) metabolite that has been most widely exploited for the in vivo investigation of metabolic processes under normal and diseased conditions [1] and is currently obtained by means of dissolution Dynamic Nuclear Polarization (d-DNP), a method that requires quite expensive and technically demanding polarizer, and is intrinsically slow.[2] ParaHydrogen Induced Polarization (PHIP) is a method for the generation of HP molecules that requires much more affordable equipment than d-DNP and it owns the great advantage that the polarization cycle takes only few minutes. The recently introduced PHIP-SAH strategy (PHIP by means of Side Arm Hydrogenation)[3] allowed to hyperpolarize pyruvate, and other metabolites, that, previously, could be obtained only by d-DNP. Aim of this work is to show the first application of PHIP hyperpolarized pyruvate to in vivo MRS-MRI metabolic studies.Methods
[1-13C] pyruvate has been hyperpolarized by means of PHIP-SAH. [3] Genetically modified Lmna mice and the corresponding WT mice were used for in vivo 13C-MRS-MRI studies, that were carried out on a 1T-MR system. 13C dynamic studies were performed and space-selective 13C-MR spectra were acquired on slices centered either on the heart or on the kidneys. 13C-CSI images were also obtained.Results
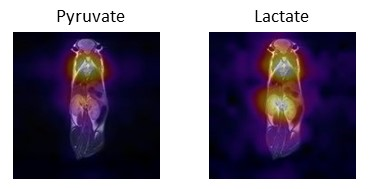
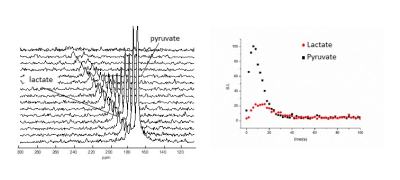
The 13C signal enhancement observed on the carboxylate moiety of [1-13C]pyruvate resulted to be 50600±5600 fold, with respect to thermal polarization at 1T, corresponding to 4.5±0.5%. 13C hyperpolarization has been back-calculated at time zero, i.e. at the end of MFC, and reached the value of 10.6±1.5 %. In order to obtain a bio-compatible aqueous solution of the HP metabolite, a diluted base (NaOH 0.1M) has been used for hydrolysis, instead of the initially reported 1M base, and methanol, used in the proof-of-concept study as hydrogenation co-solvent, has been eliminated. 13C-CSI (13C chemical shift imaging) acquisitions have been obtained within 15-20 seconds after the i.v. injection of the dose of HP pyruvate in heathy mice (wild type mice). Metabolite maps have been generated at the resonance frequencies of pyruvate and lactate (figure 1). Series of 13C-MR spectra acquired on a slab centered on heart or kidneys showed the metabolic build-up of lactate (figure 2), in both organs. Kinetic analysis of the metabolic exchange of the 13C label between [1-13C]pyruvate and [1- 13C]lactate has been carried out by monitoring the time courses of the signals of hyperpolarized lactate and pyruvate (figure 2b). The metabolic response of a WT mouse was compared to the one obtained in an analogous experiment with a Lmna mouse. The 13C HP label exchange rate between pyruvate and lactate is markedly lower in the mutant mouse than in the WT one. On the contrary, when the slice-selective 13C MR spectra are acquired on the kidneys, no difference can be observed between the transgenic Lmna and the WT mice.Discussion
Lmna mice show several diseases, called laminopaties, the most important one is cardiomyopathy,[4] i.e. a decrease of the cardiac contractile function, that is usually assessed by echocardiographic investigations. The observed reduction in the pyruvate/lactate exchange rate may be accounted for in terms of this disease. The reduced pyruvate/lactate exchange rate is a reporter of the general metabolic activity of the cells and might be due either to lower activity of the transporters (MCT) or to an altered cytosolic redox state in Lmna mice.
The 13C-MRS carried out on the kidneys allow to confirm that the altered metabolism observed in the heart of mutant mice is due to the Lmna mutations, that are limited to striatus tissues, muscles and heart, while kidneys remain unaffected.
Conclusions
This
study has shown that in vivo metabolic imaging investigations
based on the administration of a dose of [1-13C]pyruvate obtained
from the PHIP-SAH procedure is possible. The in vivo results obtained from PHIP-SAH
hyperpolarized pyruvate are consistent with the pathologic state of the heart
tissue. Consequently,
one may expect that the easy access to HP pyruvate by PHIP-SAH methodology will prompt new comers to
the use of this powerful metabolic imaging tool that was previously available
only to the d-DNP-equipped labs.Acknowledgements
This work was carried out thanks to the support of Airc (Italian Association for Cancer Research, TRIDEO call 2015) and Compagnia di San Paolo (Athenaeum Research 2016, n. CSTO164550). Scientific support from Aspect Imaging is gratefully acknowledged.References
[1] Gallagher, F. A., Kettunen, M. I. & Brindle, K. M. Biomedical applications of hyperpolarized 13C magnetic resonance imaging. Prog. Nucl. Magn. Reson. Spectrosc. 55, 285–295 (2009).
[2] Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100, 10158–63 (2003).
[3] Reineri, F., Boi, T. & Aime, S. ParaHydrogen Induced Polarization of 13C carboxylate resonance in acetate and pyruvate. Nat. Commun. 6, 5858 (2015).
[4] Arimura, T. et al. Mouse model carrying H222P- Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 14, 155–169 (2005).
Figures