0272
In vivo Imaging of Hyperpolarized Silicon-29 Nanoparticles1Institute for Biomedical Engineering, ETH & University of Zurich, Zurich, Switzerland, 2Labolatory of Physical Chemistry, ETH Zurich, Zurich, Switzerland
Synopsis
Hyperpolarized silicon particles exhibit very long T1 relaxation at room temperature, making them favourable as novel imaging MR probes. It has recently been shown that silicon particles in the nanometer size range can be efficiently polarized and image even after 4 hours upon transfer of the sample to the imaging system.
The objective of the present work was to demonstrate the imaging capability of surface functionalized hyperpolarized nanometer size silicon particles in an experimental in-vivo setting.
Introduction
The main obstacle of imaging hyperpolarized substrates is their relatively short lifetime, intrinsically limited by the T1 relaxation. Currently, most of the substrates used in vivo are based on small organic molecules (e.g. pyruvic acid) which are selectively labelled with 13C nuclei. In such a case, the imaging window span lasts around 60–90 s. This time, although short, allows to study rapid enzymatic reactions, e.g. anaerobic metabolism which can be further used to characterize tissue pathology1,2. A new class of hyperpolarized contrast agents, characterized with a long lifetime were recently proposed which are based on micro- and nanoparticles of elemental silicon. Experimental results of micro- and nanometer size silicon-29 particles3–5 revealed a lifetime of the hyperpolarized signal on the order of tens of minutes, exceeding those of any other 13C based probes reported so far. In addition, the bio-compatibility of silicon and its versatile surface chemistry makes it well suited for in vivo use6.Methods
Silicon nanoparticles: Silicon nanopowder (US Nano Research, Houston, TX, USA), synthesized from a gas phase using a laser-assisted technique, was used without further modification (average particle size (APS) ~ 55 nm). The silicon particles are characterized by an elemental purity of >99.99% and a natural abundance of 29Si of 4.7%.
Hyperpolarization of silicon: The nuclear polarization of 29Si nuclei was enhanced by dynamic nuclear polarization (DNP) exploiting endogenous defects. DNP was performed using a home-built multisample polarizer design described elsewhere7. The sample was composed of 40mg tightly packed powder enclosed in a PTFE cup placed in the polarizer.
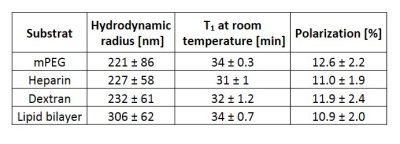
Surface functionalization: In order to improve the biodistribution and biocompatibility of the nanoparticles, the surface of the silicon nanoparticles was functionalized with NHS-dPEG4-(m-PEG12)3-ester8, Heparin9, Dextran10 or Lipid bilayer11. The hydrodynamic radius of particles was measured in phosphate buffer saline with dynamic light scattering.
MRI experiments: After 24h of continuous polarization, the samples were taken out of the polarizer and immediately transferred to a horizontal 9.4T imaging system (Bruker BioSpin, Ettlingen, Germany). A semi half-saddle surface coil (f=79 MHz) was used in T/R mode. Silicon imaging was performed using the Rapid Acquisition with Refocused Echoes (RARE) sequence with the following parameters: 64×64 encoding matrix, FOV=35×35 mm2, slice thickness 30mm, number of signal averages NA=1. Anatomical images were obtained using a T1 weighted FLASH sequence: 256×256 encoding matrix, 71% partial Fourier, TE/TR=1.8/30 ms, FOV = 40x40 mm2, slice thickness 2 mm, NA=4. All animal experiments were performed with adherence to the Swiss Animal Protection law and were approved by the regional veterinary office. Two C57BL/6 female mice (>10 weeks of age) were anesthetized with isoflurane (2%) in a mixture of O2/Air (1:4). Animals were intubated for mechanical air-ventilation (80 bpm, 25% inspiration). In addition, a 50mm long, 26G, PTFE cannula was inserted as oral gavage for intragastric (i.g.) sample injection. The silicon powder sample was dispersed in 500μl of water for injection and further flushed with 50μl of water.
Results
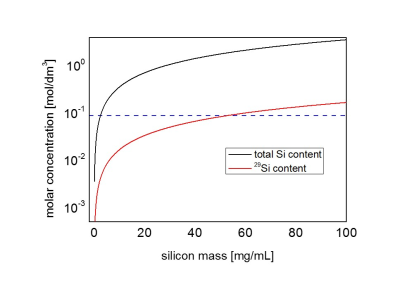
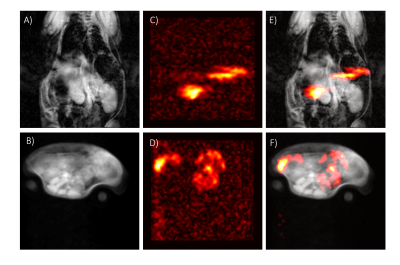
Surface functionalization with different substrates (Tab. 1) yielded similar results with respect to T1 relaxation at room temperature (31–34 min) and maximum achievable polarization (10-12%). For in-vivo imaging, 30mg of mPEG functionalized silicon nanoparticles were dispersed in 500μl for an i.g. injection, which corresponded to a concentration of ∼90mM of silicon-29 (Fig. 2). The injected hyperpolarized silicon could be successfully imaged in the two test animals in vivo with an average SNR/pixel inside the gastrointestinal tract of 12±4 and 9±2, respectively (Fig. 2 E,F).Discussion
While early reports3,5,12 of hyperpolarized silicon particles demonstrated the feasibility of imaging silicon microparticles (APS ∼2 μm), the relatively large particle size did limit in-vivo application. The present work shows that functionalized nanoparticles (APS∼55 nm) exhibit comparable relaxation and polarization properties with improved in-vivo compatibility. The signal level measured with hyperpolarized silicon-29 was found to be comparable to the signal obtained with a standard concentration of hyperpolarized [1-13C] pyruvate (80 mM) used in our laboratory. The average SNR/pixel obtained with hyperpolarized silicon was 12±4 and 9±2 in the two experiments and hence comparable to pyruvate’s downstream metabolites (lactate SNR=9.9±3.3, bicarbonate SNR= 8.9±2.5)13, despite the reduced sensitivity due to difference in gyromagnetic ratios (13Cγ/29Siγ≈1.25). Overall, good quality images were obtained despite the small amount of material used. In the future, further gains in SNR may be achieved by enriching the nanoparticles with silicon-29. Potential effects on relaxation properties, however, remain to be studied. In the example shown in this work, intragastric injection was used in the animals. In future work we will also investigate other routes of injection to study other organ systems of interest in nanomedicine applications.Acknowledgements
No acknowledgement found.References
1. Kurhanewicz J, Vigneron DB, Brindle K, et al. Analysis of cancer metabolism by imaging hyperpolarized nuclei: prospects for translation to clinical research. Neoplasia. 2011;13(2):81-97. doi:10.1593/neo.101102.
2. Rider OJ, Tyler DJ. Clinical implications of cardiac hyperpolarized magnetic resonance imaging. J Cardiovasc Magn Reson. 2013;15(1):93. doi:10.1186/1532-429X-15-93.
3. Cassidy MC, Chan HR, Ross BD, Bhattacharya PK, Marcus CM. In vivo magnetic resonance imaging of hyperpolarized silicon particles. Nat Nanotechnol. 2013;8(5):363-368. doi:10.1038/nnano.2013.65.
4. Kwiatkowski G, Jähnig F, Steinhauser J, Wespi P, Ernst M, Kozerke S. Nanometer size silicon particles for hyperpolarized MRI. Sci Rep. 2017;7(July):1-6. doi:10.1038/s41598-017-08709-0.
5. Whiting N, Hu J, Constantinou P, et al. Developing hyperpolarized silicon particles for advanced biomedical imaging applications. Proc SPIE. 2015;9417:941702. doi:10.1117/12.2082252.
6. Park J-H, Gu L, von Maltzahn G, Ruoslahti E, Bhatia SN, Sailor MJ. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat Mater. 2009;8(4):331-336. doi:10.1038/nmat2398.
7. Batel M, Krajewski M, Weiss K, et al. A multi-sample 94 GHz dissolution dynamic-nuclear-polarization system. J Magn Reson. 2012;214:166-174. doi:10.1016/j.jmr.2011.11.002.
8. Aptekar JW, Cassidy MC, Johnson a. C, et al. Hyperpolarized Long-T1 Silicon Nanoparticles for Magnetic Resonance Imaging. 2009:1-5. doi:10.1021/nn900996p.
9. Argyo C, Cauda V, Engelke H, Rädler J, Bein G, Bein T. Heparin-coated colloidal mesoporous silica nanoparticles efficiently bind to antithrombin as an anticoagulant drug-delivery system. Chem - A Eur J. 2012;18(2):428-432. doi:10.1002/chem.201102926.
10. Schulz A, Woolley R, Tabarin T, McDonagh C. Dextran-coated silica nanoparticles for calcium-sensing. Analyst. 2011;136(8):1722-1727. doi:10.1039/c0an01009j.
11. Meng H, Wang M, Liu H, et al. Use of a Lipid-Coated Mesoporous Silica Nanoparticle Platform for Synergistic Gemcitabine and Paclitaxel Delivery to Human Pancreatic Cancer in Mice. ACS Nano. 2015;9(4):3540-3557. doi:10.1021/acsnano.5b00510.
12. Whiting N, Hu J, Shah J V., et al. Real-Time MRI-Guided Catheter Tracking Using Hyperpolarized Silicon Particles. Sci Rep. 2015;5:12842. doi:10.1038/srep12842.
13. Krajewski M, Wespi P, Busch J, et al. A Multisample Dissolution Dynamic Nuclear Polarization System for Serial Injections in Small Animals. Magn Reson Med. 2017;77:904–910. doi:10.1002/mrm.26147.
Figures