0259
Cell specific anisotropy with double diffusion encoding spectroscopy in the human brain at 7T1Danish Research Centre for Magnetic Resonance, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark, 2C. J. Gorter Center for High Field MRI, Leiden University Medical Center, Leiden, Netherlands
Synopsis
The measurement of intracellular metabolite mobility with diffusion weighted spectroscopy (DWS) provides a cell-specific probe for microstructure. Measurements in animals and humans with conventional one dimensional diffusion encoding and model-guided analysis suggest that the main component of the intracellular space of both neurons and astrocytes comprises mainly anisotropic fibrous geometries. In this study we performed double diffusion encoded spectroscopy (DDES) in the human brain as a direct probe of anisotropic intracellular diffusion. As expected, our results support a main fibrous component but the results also suggest a more complex geometry of astrocytes that could include isotropic compartments.
Introduction
Unlike diffusion weighted imaging (DWI), metabolite mobility measured with diffusion weighted spectroscopy (DWS) provides a probe to cell-specific microstructure1. In particular, the intracellular diffusivity of the neuronal/axonal NAA has been demonstrated to be particularly useful for characterization of the intraneuronal/axonal space in healthy and diseased tissue2,3. In contrast to water mobility, simpler biophysical models may also be used to interpret metabolite diffusion and to obtain accurate cytomorphological information4–7. We recently leveraged those findings to introduce powder averaged DWS to account for the unknown macroscopic orientational distribution of fibers8. Those analyses, however, assume that disperse anisotropic domains fully account for the non-monoexponential signal attenuation in a single diffusion encoding (SDE) sequence. Double diffusion encoding (DDE) or multidimensional diffusion encoding (MDE) overcomes this assumption and provides a direct probe to underlying microscopic anisotropy in disordered tissues9,10. The cosinusoidal signal modulation with respect to the relative angle θ between two separated diffusion weighting gradient encodings is the hallmark of microscopic anisotropy11. The method has recently been adopted for imaging using different approaches and enables the measurement of microscopic fractional anisotropy (µFA)12,13. µFA is related to the conventional FA value from DTI analysis but unbiased by the underlying macroscopic fiber architecture. DDE was recently combined with DWS in mice and demonstrated a strong underlying anisotropy in all brain metabolites14. In this work we apply double diffusion encoding spectroscopy (DDES) in human studies.Methods
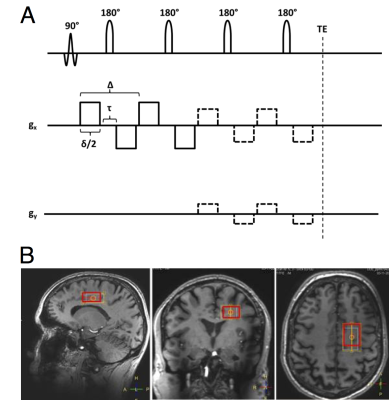
Data acquisition: Experiments were performed on 5 healthy volunteers (41 ± 19 y/o). Data were acquired on a Philips 7T whole body MRI scanner (Philips, Cleveland OH, USA), equipped with a volume transmit/32-channel receive head coil ((Nova Medical, Wilmington MA, USA)). The sLASER-based DDSE sequence diagram is shown in (fig 1A). A volume of interest of 3(A-P)×2(R-L)×1.5(F-H) cm3 was positioned on a parietal region containing mostly white matter (fig 1B). TR/TE = 5000/185ms. For each diffusion weighting block, the single gradient duration was (δ/2) = 15.5 ms, bipolar gap (τ) = 10 ms and Δ = 45 ms. The direction of the first gradient was set to [1,1,-0.5] and the second gradient was given in 12 equally spaced directions, tracing a circle that included [1,1,-0.5]. The resulting b was 7192 s/mm2, and each condition was repeated 24 times for a total acquisition time of 25 minutes. Analysis: The individual spectra were corrected for eddy currents and phase and frequency variations. Water, tNAA, pCho and tCr levels were quantified for b=0 and at b=7192 s/mm2 for each θ value using LCmodel15. We initially assume fully disperse Gaussian domains and fitted the θ modulation from an ensemble of uniformly rotated axisymmetric diffusion tensors to the normalized data with respect to the longitudinal and transversal diffusivities DL and DT. µFA was calculated as a conventional FA based on those diffusivities.Results
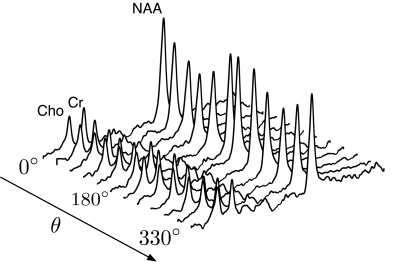
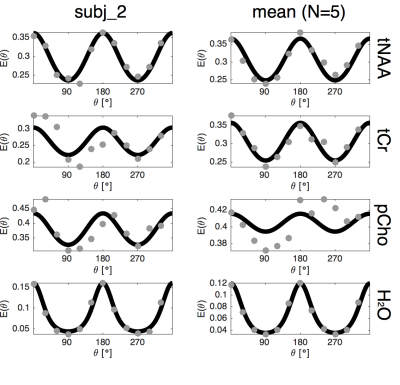
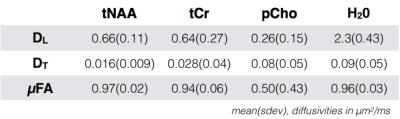
The tNAA, pCho and tCr peaks were clearly visible in all spectra with a cosinusoidal θ modulation with quantifiable peaks within a CRLB up to 8% (fig 2). The normalized metabolite levels and the model fit are shown for a single subject and the group mean in fig 3. Fitted model parameters (DL, DT and derived µFA) are shown in fig 4.Discussion and Conclusion
Metabolite diffusivities were comparable to values presented earlier with SDE in mice and human7,8 and DDE in mice16. The astrocytic PCho diffusivity showed somewhat lower µFA compared to the neuronal tNAA signal, suggesting an additional contribution from isotropic cell bodies in white matter that could provide a potential marker of disease progression, e.g. of glial reactivity in response to neuroinflammation. Water µFA was close to the tNAA values reflecting that fast extracellular components are filtered out at this relatively high b-value. We deliberately placed the VOI in a region with known high fiber dispersion but effects from residual macroscopic anisotropy cannot be fully excluded. This can be addressed with model approaches or extended gradient orientation schemes to provide more robust powder averages17,18. Time dependent effects should be considered at the short mixing times used in our setup and would be manifested as a signal difference between the parallel and antiparallel conditions θ = 0° and 180°. This was not observed and we thus expect those effects to be small with the current gradient timings. However, this effect could provide additional information and can be explored with a range of mixing times or tuned/detuned gradient pulses as previously done in DDE and MDE settings19,20. We will in future experiments also consider a range of b-values to fully separate variances related to anisotropy and isotropic heterogeneity21.Acknowledgements
This work is supported by the Danish Council for Independent Research (4093-00280A and 4093-00280B).References
1. Ronen I, Valette J. Diffusion-weighted magnetic resonance spectroscopy. eMagRes. 2015;4(4):733-750
2. Wood ET, Ronen I, Techawiboonwong A, et al. Investigating Axonal Damage in Multiple Sclerosis by Diffusion Tensor Spectroscopy. J Neurosci. 2012;32(19):6665-6669
3. Branzoli F, Ercan E, Valabr??gue R, et al. Differentiating between axonal damage and demyelination in healthy aging by combining diffusion-tensor imaging and diffusion-weighted spectroscopy in the human corpus callosum??at??7??T. Neurobiol Aging. 2016;47:210-217
4. Najac C, Branzoli F, Ronen I, Valette J. Brain intracellular metabolites are freely diffusing along cell fibers in grey and white matter, as measured by diffusion-weighted MR spectroscopy in the human brain at 7 T. Brain Struct Funct. 2016;221(3):1245-1254
5. Ronen I, Ercan E, Webb A. Axonal and glial microstructural information obtained with diffusion-weighted magnetic resonance spectroscopy at 7T. Front Integr Neurosci. 2013;7:1-10
6. Kroenke CD, Ackerman JJH, Yablonskiy DA. On the nature of the NAA diffusion attenuated MR signal in the central nervous system. Magn Reson Med. 2004;52(5):1052-1059
7. Palombo M, Ligneul C, Valette J. Modeling diffusion of intracellular metabolites in the mouse brain up to very high diffusion-weighting: Diffusion in long fibers (almost) accounts for non-monoexponential attenuation. Magn Reson Med. 2017;77(November 2016):343-350.
8. Lundell H, Ingo C, Dyrby TB, Ronen I. Accurate estimation of intra-axonal di ff usivity and anisotropy of NAA in humans at 7T study we address the problem of macroscopic dispersion of fi ber directions and suggest the use of high angular gradient resolution and powder averaging as an experime. Proc Intl Soc Mag Reson Med. 2017;25:1083.
9. Shemesh N, Jespersen SN, Alexander DC, et al. Conventions and nomenclature for double diffusion encoding NMR and MRI. Magn Reson Med. 2016;75(1):82-87
10. Topgaard D. Multidimensional diffusion MRI. J Magn Reson. 2016;275:98-113
11. Mitra PP. Multiple wave-vector extensions of the NMR pulsed-field-gradient spin-echo diffusion measurement. Phys Rev B. 1995;51(21).
12. Jespersen SN, Lundell H, Sønderby CK, Dyrby TB. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed. 2013.
13. Lasič S, Szczepankiewicz F, Eriksson S, Nilsson M, Topgaard D. Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Front Phys. 2014;2(February):1-14.
14. Shemesh N, Rosenberg JT, Dumez J-NJ-N, Muniz JA, Grant SC, Frydman L. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat Commun. 2014;5:4958
15. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001
16. Shemesh N, Rosenberg JT, Dumez JN, Grant SC, Frydman L. Distinguishing neuronal from astrocytic subcellular microstructures using in vivo Double Diffusion Encoded 1H MRS at 21.1 T. PLoS One. 2017;12(10):1-19.
17. Jespersen SN, Lundell H, Sønderby CK, Dyrby TB. Orientationally invariant metrics of apparent compartment eccentricity from double pulsed field gradient diffusion experiments. NMR Biomed. 2013;26(12):1647-1662.
18. Shemesh N, Barazany D, Sadan O, et al. Mapping apparent eccentricity and residual ensemble anisotropy in the gray matter using angular double-pulsed-field-gradient MRI. Magn Reson Med. 2012;68(3):794-806.
19. Özarslan E, Basser PJ. Microscopic anisotropy revealed by NMR double pulsed field gradient experiments with arbitrary timing parameters. J Chem Phys. 2008
20. Lasič S, Lundell H, Topgaard D, Dyrby TB. Effects of imaging gradients in sequences with varying longitudinal storage time-Case of diffusion exchange imaging. Magn Reson Med. 2017;0:1-8
21. Szczepankiewicz F, Lasič S, van Westen D, et al. Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: Applications in healthy volunteers and in brain tumors. Neuroimage. 2015
Figures