0258
On the magnetic field and echo time dependence of the pseudo-diffusion coefficient1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom, 3Department Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Center for Medical Physics and Engineering, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5CUBRIC, School of Psychology, Cardiff University, Cardiff, United Kingdom
Synopsis
It has been reported that a strong echo time dependence of the perfusion fraction f exists in liver and pancreas. The purpose of this work is to investigate whether a similar dependence exists for the pseudo-diffusion coefficient D*. Thereto, the livers of six healthy volunteers were examined at two echo times (TE = 40/80 ms) at two field strengths. In contrast to f, D* shows almost no echo time dependence in the healthy liver and the observed field strength dependence was smaller than the fit uncertainty.
Purpose
Intravoxel incoherent motion (IVIM) diffusion weighted imaging has so far been applied in numerous studies, for example, for characterizing liver diseases1. The IVIM model promises to deliver quantitative parameters that can be used for comparative studies performed by different investigators with potentially non-identical acquisition parameters. Concerning the perfusion fraction f, Lemke et al.2 showed that a strong echo time dependence exists in liver and pancreas, which must be taken into account when comparing data acquired with differing acquisition parameters. The purpose of this work is to investigate whether a similar dependency exists for the pseudo-diffusion coefficient D*. Such a dependency might, for example, originate from different signal contributions of arterial and venous blood, which are known to have different and field-dependent T2 relaxation times3,4.Methods
Measurements were performed with a modified spin echo echo planar imaging sequence implemented on a 1.5-Tesla (Magnetom Aera, Siemens, Erlangen, Germany) and on a 3-Tesla whole-body MR system (Magnetom Skyra, Siemens, Erlangen, Germany). The livers of six healthy volunteers were examined at two echo times (TE = 40/80 ms) at the two field strengths. The repetition time was set to 2000 ms, voxel size to 3.44 x 3.44 mm², slice thickness to 5 mm and the matrix size to 100x66. Partial Fourier factor of 75% and a bandwidth of 2780 Hz/Px was used. The used b-value distribution was optimized using published IVIM parameter values resulting in the choice of 15 b-values (0.6, 1.4, 2.0, 2.9, 3.4, 4.1, 5.2, 12.0, 20.4, 25.6, 28.4, 35.2, 41.2, 59.0, 72.0 s/mm²). For TE = 80 ms, the additional b-value 600 s/mm² was used for estimation of the diffusion coefficient. The influence of imaging gradients on b-values was taken into account. For each b-value and echo time, one measurement block consisting of six weighted and four unweighted images was acquired during exhaled breath hold.
For data analysis, a polygonal region of interest (ROI) was defined in a trusted region without large vessels for each measurement block. For each b-value and volunteer, the ROI’s median was used for calculating mean and standard deviation of all volunteers. The bi- and tri-exponential IVIM model were fitted to the data. The diffusion coefficient was fixed to the value determined at TE = 80 ms with a mono-exponential fit using higher b-values (b>50 s/mm²).
Results and Discussion
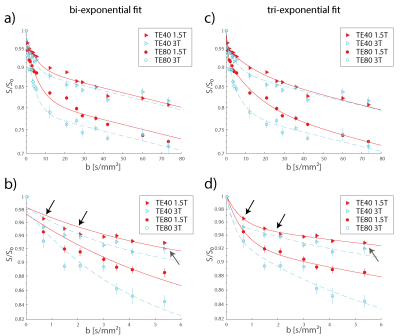
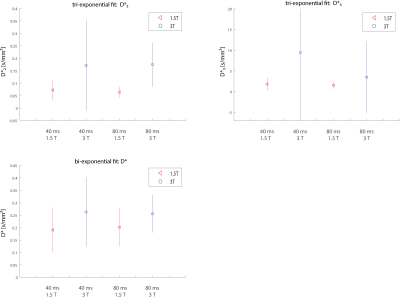
Figure 1 shows the signal dependence on the b-value. Data at lower b-values (b<10 s/mm²) is better matched by the tri-exponential fit compared to the bi-exponential one. This might be explained by the presence of another diffusion compartment or by the velocity distribution of individual compartments. It confirms the findings of the recently published articles (kidney5,6, liver6,7) that have reported on the tri-exponential signal decay. For the bi-exponential model, the pseudo-diffusion coefficient D* was found to be 0.191/0.200 mm²/s at 1.5 T and 0.263/0.256 mm²/s at 3 T with TE = 40/80 ms. For the slow pseudo-diffusion compartment in the tri-exponential model, the pseudo-diffusion coefficient D*2 was determined as 0.072/0.064 mm²/s at 1.5 T and 0.172/0.175 at 3 T at TE = 40/80 ms. For the fast compartment, the pseudo-diffusion coefficient D*3 was determined as 1.821/1.576 mm²/s at 1.5 T and 9.449/3.515 mm²/s at 3 T at TE = 40/80 ms.
Figure 2 shows the pseudo-diffusion coefficients determined from the group-averaged signal curves. An echo time dependence cannot be observed for any of the pseudo-diffusion coefficients. The mean values of all pseudo-diffusion coefficients increase at 3 T. However, this increase is smaller than the size of the large confidence intervals at 3 T so that a definite statement about field-strength dependency cannot be made based on the current data. Increased pseudo-diffusion coefficients at 3 T would not be unreasonable, given the published values of T2 times of arterial and venous blood3, 4, which predict that the signal contribution of arterial blood should rise with field strength.
Conclusion
In contrast to f, D* shows almost no echo time dependence in the healthy liver and the observed field strength dependency was smaller than the fit uncertainty. Thus D* appears to have the favorable property of being less dependent on acquisition parameters than f, although a main problem remains the fit uncertainty of D*.Acknowledgements
Financial support by the DFG (grant number LA 2804/6-1) is gratefully acknowledgedReferences
1. Li YT, Cercueil JP, Yuan J et al. Liver intravoxel incoherent motion (IVIM) magnetic resonance imaging: a comprehensive review of published data on normal values and applications for fibrosis and tumor evaluation. Quantitative imaging in medicine and surgery 2017;7(1):59-78.
2. Lemke A, Laun FB, Simon D, et al. An in vivo verification of the intravoxel incoherent motion effect in diffusion-weighted imaging of the abdomen. Magnetic resonance in medicine 2010;64(6):1580-1585.
3. Zhao JM, Clingman CS, Narvainen MJ, et al. Oxygenation and hematocrit dependence of transverse relaxation rates of blood at 3T. Magnetic resonance in medicine 2007;58(3):592-597.
4. Silvennoinen MJ, Clingman CS, Golay X, et al. Comparison of the dependence of blood R2 and R2* on oxygen saturation at 1.5 and 4.7 Tesla. Magnetic resonance in medicine 2003;49(1):47-60.
5. Van der Bel R, Gurney-Champion OJ, Froeling M, et al. A tri-exponential model for intravoxel incoherent motion analysis of the human kidney: In silico and during pharmacological renal perfusion modulation. European journal of radiology 2017;91:168-174.
6. Wurnig MC, Germann M, Boss A. Is there evidence for more than two diffusion components in abdominal organs? - A magnetic resonance imaging study in healthy volunteers. NMR in biomedicine 2017
7. Cercueil JP, Petit JM, Nougaret S, et al. Intravoxel incoherent motion diffusion-weighted imaging in the liver: comparison of mono-, bi- and tri-exponential modelling at 3.0-T. Eur Radiol 2015;25(6):1541-1550.
Figures