0257
The "Magic DIAMOND" method: probing brain microstructure by combining b-tensor encoding and advanced diffusion compartment imaging1Université de Sherbrooke, Sherbrooke, QC, Canada, 2Dept. of Radiology, Boston Children’s Hospital, Boston, MA, United States, 3MR Clinical Science, Philips Healthcare Canada, Markham, ON, Canada, 4Department of Clinical Sciences, Lund, Lund University, Lund, Sweden, 5Random Walk Imaging AB, Lund, Sweden
Synopsis
Via q-trajectory encoding, b-tensors enable the disentanglement of isotropic and anisotropic diffusion components. Relevant metrics are usually extracted from data acquired with a combination of linear and spherical b-tensors with 1D parametric distributions of diffusivities. Independently, the DIAMOND model proposed an analytic result for a 6D parametric compartmental tensor distribution based on linearly acquired data. In this work, we extend DIAMOND’s analyticity to axisymmetric acquisitions. Evaluating this "Magic DIAMOND" approach on in vivo data, we show that it can tease apart isotropic diffusion and diffusivity compartments of crossing fascicles, hereby integrating specific compartments with intra-compartment diffusional variance.
Context and purpose
B-tensor encoding is an emerging concept that allows isotropic and anisotropic components of the diffusion-weighted signal to be disentangled1-4. This concept has already improved interpretation of diffusion MRI results in healthy and tumor tissue5,6, as well as in chronic schizophrenia7. It has also been used8 to test and invalidate assumptions in microstructure models such as NODDI9.
The idea of an intra-voxel diffusion tensor distribution (DTD) emerges naturally from b-tensor encoding10. For instance, Westin et al. considered a cumulant approach7 that implicitly assumes a normal distribution of diffusion tensors. Independently, Scherrer et al. developed the DIAMOND model11, whose refined version12 describes the DTD through the analytically tractable non-central matrix-variate Gamma distribution13-15. While Gamma distributions are better than many other distributions for modelling 1D distributions16, their matrix-variate alternative models an asymmetric distribution of diffusion tensors in the space $$$\mathrm{Sym}^+(3)$$$ of symmetric positive-definite random diffusion matrices. This versatile distribution fitting explains why DIAMOND retains greater specificity from typical linear acquisitions12.
In this work, we leverage b-tensor encoding and the DIAMOND model to propose the first b-tensor-dependent 6D parametric distribution model for diffusion compartment imaging (DCI). We demonstrate that this method, dubbed the "Magic DIAMOND" method after the magic angle spinning acquisition for spherical encoding1, has an analytic solution and evaluate fascicle metrics from in vivo data.
Theory
Within DCI, the signal acquired through b-tensor encoding is given by:$$\mathcal{S}(\mathbf{b})=\mathcal{S}_0\sum_{j=1}^{N_\text{c}}\int_{\mathbf{D}\in\mathrm{Sym}^+(3)}\!\mathcal{P}_j(\mathbf{D})\,\exp(-\mathbf{b}:\mathbf{D})\,\mathrm{d}\mathbf{D}\,,$$where $$$N_\text{c}$$$ is the number of compartments, $$$\mathcal{P}_j(\mathbf{D})$$$ is the $$$j$$$th compartment's DTD, $$$\mathbf{b}=\int_0^\tau\!q^2(t)\,\mathbf{n}(t)\cdot\mathbf{n}^\text{T}(t)$$$ is the b-tensor given by the trajectory over diffusion time $$$\tau$$$ of the spin-dephasing vector $$$\mathbf{q}(t)=q(t)\,\mathbf{n}(t)$$$, and$$\mathbf{b}:\mathbf{D}=\int_0^\tau\!q^2(t)\,\mathbf{n}^\text{T}(t)\cdot\mathbf{D}\cdot\mathbf{n}(t)\,\mathrm{d}t=\sum_{ij}b_{ij}\,D_{ij}$$is the Frobenius inner product.
Omitting compartment index, DIAMOND12 considers the non-central matrix-variate Gamma distribution13-15:$$\mathcal{P}_{\kappa,\bf{\Psi},\bf{\Theta}}(\mathbf{D})=\frac{\mathrm{Det}(\mathbf{D})^{\kappa-2}}{\mathrm{Det}(\mathbf{\Psi})^\kappa\,\Gamma_3(\kappa)}\,\exp\!\left[-\mathrm{Tr}(\mathbf{\Theta}+\mathbf{\Psi}^{-1}\cdot\mathbf{D})\right]\mathcal{F}_{0,1}(\kappa,\mathbf{\Theta}\cdot\mathbf{\Psi}^{-1}\cdot\mathbf{D})\,,$$where $$$\kappa>1$$$ and $$$\mathbf{\Psi}\in\mathrm{Sym}^+(3)$$$ are the shape and scale parameters, $$$\mathcal{F}_{0,1}$$$ is the hypergeometric function of $$$(0,1)$$$-order matrix argument, $$$\Gamma_3(\kappa)=\pi^{3/2}\prod_{p=1}^3\Gamma(\kappa-(p-1)/2)$$$ is the multivariate Gamma function, and $$$\mathbf{\Theta}\in\mathrm{Sym}(3)$$$ is the noncentrality parameter that allows for an asymmetric distribution of diffusion tensors in $$$\mathrm{Sym}^+(3)$$$. It can be shown13 that this distribution's moment-generating function writes$$M_\mathbf{D}(\mathbf{Z})=\int\!\mathcal{P}(\mathbf{D})\,\exp^{\mathrm{Tr}(\mathbf{Z}\cdot\mathbf{D})}\,\mathrm{d}\mathbf{D}=[\mathrm{Det}(\mathbf{I}_3-\mathbf{Z}\cdot\mathbf{\Psi})]^{-\kappa}\;\mathrm{exp}\!\left[\mathrm{Tr}([(\mathbf{I}_3-\mathbf{Z}\cdot\mathbf{\Psi})^{-1}-\mathbf{I}_3]\cdot\mathbf{\Theta})\right]$$for $$$\mathbf{Z}$$$ satisfying $$$(\mathbf{I}_3-\mathbf{Z}\cdot\mathbf{\Psi})\in\mathrm{Sym}^+(3)$$$. While linear encoding of b-value $$$b$$$, oriented along $$$\mathbf{n}$$$, yields the signal $$$\mathcal{S}(b)=\mathcal{S}_0\,M_\mathbf{D}(-b\,\mathbf{n}\cdot\mathbf{n}^\text{T})$$$, any b-tensor encoding is encapsulated in$$\mathcal{S}(\mathbf{b})=\mathcal{S}_0\,M_{\mathbf{D}}(-\mathbf{b})=\mathcal{S}_0\,M_{\mathbf{D}}\left(-\int_0^\tau\!q^2(t)\,\mathbf{n}(t)\cdot\mathbf{n}^\text{T}(t)\,\mathrm{d}t\right)\,,$$with b-value $$$b=\mathrm{Tr}(\mathbf{b})=\int_0^\tau\!q^2(t)\,\mathrm{d}t$$$. Combining the Woodbury matrix identity, the linearity of the trace, and the matrix determinant lemma gives$$\mathcal{S}(\mathbf{b})=\mathcal{S}_0\,[\mathrm{Det}(\mathbf{I}_3+\mathbf{\Psi}\cdot\mathbf{b})]^{-\kappa}\;\mathrm{exp}\!\left[-\mathbf{b}:[(\mathbf{I}_3+\mathbf{\Psi}\cdot\mathbf{b})^{-1}\cdot\mathbf{\Psi}\cdot\mathbf{\Theta}]\right]\,.$$For axisymmetric diffusion, the average compartment tensor ($$$\mathcal{P}_{\kappa,\bf{\Psi},\bf{\Theta}}$$$'s expectation) writes$$\mathbf{D}^0=\mathbf{\Psi}\cdot(\kappa \mathbf{I}_3+\mathbf{\Theta})=\mathbf{V}\cdot\mathrm{Diag}(\lambda^\perp,\lambda^\perp,\lambda^\parallel)\cdot\mathbf{V}^{\mathrm{T}}\,,$$where $$$ \lambda^\perp\leq\lambda^\parallel$$$ and $$$\mathbf{V}$$$ is the diffusion eigenmatrix containing orientations, and the noncentrality parameter$$\mathbf{\Theta}=\mathbf{V}\cdot\mathrm{Diag}(0,0,\kappa^\prime)\cdot\mathbf{V}^{\mathrm{T}}\,,$$where $$$\kappa^\prime$$$ is an additional shape parameter. Defining $$$\kappa^\parallel=\kappa+\kappa^\prime$$$ and $$$\kappa^\perp=\kappa$$$) and writing the axisymmetric b-tensor$$\mathbf{b}=\mathbf{W}\cdot\left[\frac{b_\text{S}}{3}\,\mathrm{Diag}(1,1,1)+b_\text{L}\,\mathrm{Diag}(0,0,1)\right]\cdot\mathbf{W}^\text{T}\,,$$where $$$\mathbf{W}$$$ is $$$\mathbf{b}$$$'s eigenmatrix and10$$(b_S,b_L)=\begin{cases}(b,0)&\text{(spherical encoding)}\\(0,b)&\text{(linear encoding)}\\(3b/2,-b/2)&\text{(planar encoding)}\end{cases}\,,$$we obtain the general Magic DIAMOND signal model$$\begin{eqnarray}\mathcal{S}(b_\text{S},b_\text{L},\beta)=&\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\!\mathcal{S}_0\left[\left(1+\frac{b_\text{S}}{3}\,\frac{\lambda^\parallel}{\kappa^\parallel}\right)\left(1+\frac{b_\text{S}}{3}\, \frac{\lambda^\perp}{\kappa^\perp} \right)^2\right.\\ & \left.+\,b_\text{L}\left(1+\frac{b_\text{S}}{3}\,\frac{\lambda^\perp}{\kappa^\perp}\right)\left(\frac{\lambda^\parallel}{\kappa^\parallel}\cos^2\beta+\frac{\lambda^\perp}{\kappa^\perp}\sin^2\beta+\frac{b_\text{S}}{3}\,\frac{\lambda^\parallel\lambda^\perp}{\kappa^\parallel\kappa^\perp}\right)\right]^{-\kappa_\perp}\\ &\times\mathrm{exp}\!\left[\frac{\displaystyle-\left[\frac{b_\text{S}}{3}+b_\text{L}\cos^2\beta+\frac{b_\text{S}}{3}\left(\frac{b_\text{S}}{3}+b_\text{L}\right)\frac{\lambda^\perp}{\kappa^\perp}\right]\frac{\kappa^\parallel-\kappa^\perp}{\kappa^\parallel}\,\lambda^\parallel}{\displaystyle\left(1+\frac{b_\text{S}}{3}\frac{\lambda^\parallel}{\kappa^\parallel}\right)\left(1+\frac{b_\text{S}}{3}\,\frac{\lambda^\perp}{\kappa^\perp}\right)+b_\text{L}\left(\frac{\lambda^\parallel}{\kappa^\parallel}\cos^2\beta+\frac{\lambda^\perp}{\kappa^\perp}\sin^2\beta+\frac{b_\text{S}}{3}\,\frac{\lambda^\parallel\lambda^\perp}{\kappa^\parallel\kappa^\perp} \right)}\right]\end{eqnarray}$$with Euler angle $$$\beta$$$ separating the main axes of $$$\mathbf{b}$$$ and $$$\mathbf{D}^0$$$.
Although planar encoding is not yet available on our MRI scanner, we also prove that$$\ln\!\left(\frac{\mathcal{S}_\text{linear}(b,\beta)}{\mathcal{S}_\text{planar}(2b,\beta\pm\pi/2)}\right)=\kappa^\perp\ln\!\left(1+b\,\frac{\lambda^\perp}{\kappa^\perp}\right)\,,$$which $$$\beta$$$-independently probes compartmental radial heterogeneities of the DTD.
Methods
MRI acquisitions were performed on a clinical 3T system with 45 mT/m maximum gradient amplitude (Ingenia, Philips Healthcare, Best, the Netherlands) using a 32-channel head coil. Imaging was performed using a prototype diffusion-weighted spin-echo EPI sequence with numerically optimized17 and spectrally matched18 spherical and linear encoding waveforms. Acquisition parameters were: TR=12000 ms, TE=128 ms, spatial resolution=2x2x4 mm3, 30 slices, SENSE factor=1.9, multi-shell scheme with 1x$$$b$$$=0 s/mm2, 6x$$$b$$$=100 s/mm2, 6x$$$b$$$=700 s/mm2, 12x$$$b$$$=1400 s/mm2 and 20x$$$b$$$=2000 s/mm2, "standard parameters" that have proven efficient in investigations of powder-averaged signals19.
The diffusion-weighted images were resampled to 2x2x2 mm3 using sinc interpolation. We estimated DIAMOND from the linear data and Magic DIAMOND from the spherical and linear data. We investigated the compartments' orientations and the free water fraction (FW), and compared the fascicle microscopic FA (f$$$\mu$$$FA) of Magic DIAMOND to the fascicle FA (fFA) of DIAMOND. Finally, we achieved multi-peak tractography of the arcuate fasciculus and colored the tract streamlines using the f$$$\mu$$$FA of the anisotropic compartment most aligned with the local streamline orientation.
Results and discussion
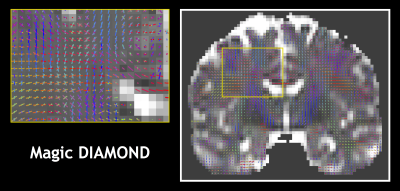
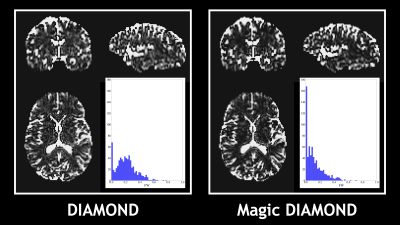
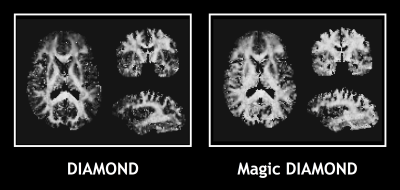
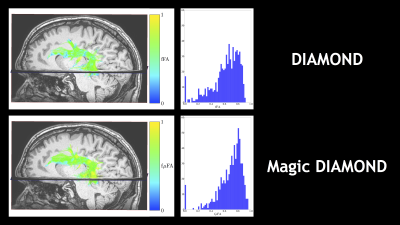
Fig.1 shows that Magic DIAMOND orientations of the compartmental expectations $$$\mathbf{D}^0$$$ are consistent with the expected anatomy. Fig.2 demonstrates that the estimated FW in the white matter is smaller with Magic DIAMOND, as a consequence of the DIAMOND estimation being more constrained by spherical encoding data. Fig.3 shows that the maximal f$$$\mu$$$FA map resembles a T1-weighted image, as expected. Finally, Fig.4 exhibits a multi-peak tractography of the arcuate fasciculus whose color map indicates that Magic DIAMOND's streamlines are longer since f$$$\mu$$$FA is less vulnerable to partial volume effects in the grey matter.Conclusion
We show that advanced diffusion compartment imaging can incorporate b-tensor encoding. Moreover, our analysis foresees an important use of planar encoding, combined with linear encoding: specifically estimating compartmental radial heterogeneities of the DTD. Finally, the imaging applications of the Magic DIAMOND formulation, although preliminary, shows the potential of estimating diffusion tensor distributions in complex multi-fascicle environments.Acknowledgements
M. Descoteaux was supported by his NSERC Discovery grant and the NeuroInformatics USherbrooke Institutional Research Chair.
B. Scherrer was supported in part by the National Institutes of Health (NIH) grants R01 NS079788, U01 NS082320 and by Boston Children's Hospital Innovator Award.
References
1. S. Eriksson et al., Isotropic diffusion weighting in PGSE NMR by magic-angle spinning of the q-vector, Journal of Magnetic Resonance 226, 13 (2013).
2. S. Lasič et al., Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector, Frontiers in Physics 2, 11 (2014).
3. S. Eriksson et al., NMR diffusion-encoding with axial symmetry and variable anisotropy: Distinguishing between prolate and oblate microscopic diffusion tensors with unknown orientation distribution, The Journal of Chemical Physics 142, 104201 (2015).
4. D. Topgaard, Multidimensional diffusion MRI, Journal of Magnetic Resonance 275, 98 (2017).
5. F. Szczepankiewicz et al., Quantification of microscopic diffusion anisotropy disentangles effects of orientation dispersion from microstructure: Applications in healthy volunteers and in brain tumors, NeuroImage 104, 241 (2015).
6. F. Szczepankiewicz et al., The link between diffusion MRI and tumor heterogeneity: Mapping cell eccentricity and density by diffusional variance decomposition (DIVIDE), NeuroImage 142,522 (2016).
7. C. F. Westin et al., Q-space trajectory imaging for multidimensional diffusion MRI of the human brain, NeuroImage 135, 345 (2016).
8. B. Lampinen et al., Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding, NeuroImage 147, 517 (2017).
9. H. Zhang et al., NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain, NeuroImage 61, 1000 (2012).
10. J. a. P. de Almeida Martins and D. Topgaard, Two-Dimensional Correlation of Isotropic and Directional Diffusion Using NMR, Phys. Rev. Lett. 116, 087601 (2016).
11. B. Scherrer et al., Characterizing brain tissue by assessment of the distribution of anisotropic microstructural environments in diffusion-compartment imaging (DIAMOND), Magnetic Resonance in Medicine 76, 963 (2016).
12. B. Scherrer et al., Decoupling Axial and Radial Tissue Heterogeneity in Diffusion Compartment Imaging (Springer International Publishing, Cham, 2017), pp. 440–452.
13. R. J. Muirhead, Aspects of Multivariate Statistical Theory (John Wiley and Sons Ltd., Hoboken, New Jersey, 1982).
14. A. K. Guptar and D. K. Nagar, Matrix Variate Distributions (Chapman and Hall/CRC, Boca Raton, Florida, 2000).
15. T. H. Anderson, An Introduction to Multivariate Statistical Analysis (Third Edition, John Wiley and Sons Ltd., Hoboken, New Jersey, 2003).
16. M. Röding et al., Gamma convolution models for self-diffusion coefficient distributions in PGSE NMR. Journal of Magnetic Resonance, 261, 6-10 (2015).
17. J. Sjölund et al., Constrained optimization of gradient waveforms for generalized diffusion encoding, Journal of Magnetic Resonance 261, 157-168 (2015).
18. H. Lundell et al., Microscopic anisotropy with spectrally modulated q-space trajectory encoding. Int. Soc. Magn. Reson. Med. Honolulu, Hawaii, (2017).
19. F. Szczepankiewicz et al., Whole-brain diffusional variance decomposition (DIVIDE) in 8 minutes: Technical feasibility at 1.5, 3, and 7 T. In: Proc. Intl. Soc. Mag. Reson. Med. 25, 2017 Honolulu, USA. 3503, (2017).
Figures