0239
Preoperative Remnant Liver Function Evaluation using a Clinical-Available Gd-EOB-DTPA-Enhanced MR Imaging Protocol in HCC Patients1Center for Biomedical Imaging Research, School of Medicine, Tsinghua University, Beijing, China, 2Department of Radiology, Southwest Hospital, Third Military Medical University, Chongqing, China, 3Beijing Tsinghua Changgung Hospital, School of Medicine, Tsinghua University, Beijing, China
Synopsis
Accurate evaluating of remnant liver function preoperatively is important for surgery planning and reducing posthepatectomy liver failure (PHLF) rate in hepatocellular carcinoma (HCC) patients. In this study, an accurate remnant liver function evaluation method was proposed using a clinical-available MR imaging protocol. The remnant liver function measured by the proposed method showed significant difference between the patients with and without PHLF. More importantly, ROC analysis showed the proposed method has a larger AUC than remnant liver volume and ICG based parameters in predicting PHLF.
Introduction
Liver cancer is the second leading cause of death among all cancers worldwide1. Resection is the mainstream of liver cancer treatment2 but patients may still suffer from posthepatectomy liver failure (PHLF)3,4 due to the insufficient liver function. Accurate evaluating of remnant liver function preoperatively is important for surgery planning and reducing PHLF rate5. In current clinical practice, serum based tests were used to rule out the high-risk patients of PHLF6,7, but their accuracy is limited because they only reflect the whole liver function, not the localized remnant function. Recently, methods based on SPECT8,9 or DCE-MRI with liver-specific contrast agents10,11 have been proposed to image the localized liver function. However, these new imaging techniques have poor clinical availability. In this study, an accurate remnant liver function evaluation method based on a clinical available imaging protocol was proposed and its value in predicting PHLF was tested in a retrospective study.Methods
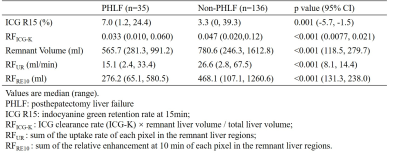
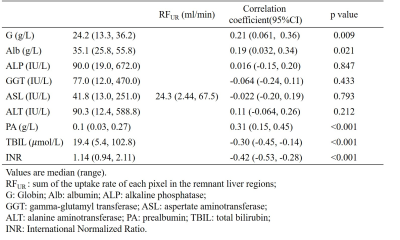
This retrospective study was approval by the Ethics Committee. 171 HCC patients (151 male, age median 50, range 19~77) who underwent liver resection between 2015 and 2017 were selected. MR scans were performed on a 3T Siemens MR scanner using a Sense Body Coil before the liver resection. T1-weighted 3D volume-interpolated body examination (VIBE) images were acquired before and at the time when the arcus aortae were enhanced, 70 s, 180 s, 5 min, and 10 min after the administration of 0.025 mmol/kg Gd-EOB-DTPA at a rate of 2 ml/s. The scan parameters were as follows: TR=3.42 ms, TE=1.25 ms, FA=10o, FOV=275*400 mm2, slice thickness=2 mm, slice=96, in-plane resolution=1.25*1.25 mm2. First, all the phases were registered. Then the liver function parameters were calculated for each pixel of liver parenchyma, including the uptake rate (UR, /min) by a dual-inlet two-compartment Patlak model12, and the relative enhancement at 10min (RE10)13 after normalization of the image intensity with muscle signal as reference. The remnant liver regions after resection were defined semi-automatically using IQQA®-3D (EDDA Technology, China) according to the operation records. Then the remnant liver function (RFUR (ml/min), RFRE10 (ml)) can be defined by sum of the liver function parameters (UR, RE10) of each pixel in the remnant liver regions. The indocyanine green (ICG) retention rate at 15 min (ICG R15) before the liver resection within a week was also accessed as a current standard liver function parameter. As a comparison, a preoperative remnant liver function assessed by ICG retention rate was defined as RFICG-K = ICG clearance rate (ICG-K) × remnant liver volume / total liver volume13. The blood serum indices including G, Alb, ALP, GGT, AST, ALT, PA, INR and TBIL were accessed on postoperative day 5. The PHLF was defined according to the International Study Group of Liver Surgery (ISGLS)14. The continuous variables of the patients with and without PHLF were compared using the two-tailed independent Student’s t test. Receiver operating characteristics (ROC) analysis was applied and the area under the ROC curves (AUC) was calculated. The best cut-off value was chosen to maximize the Youden’s index. The Pearson or Spearman correlation coefficient was calculated between the imaging parameters and the postoperative blood serum indices.Results
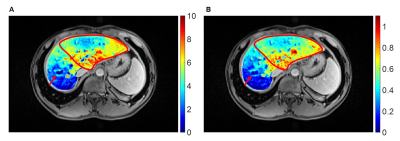
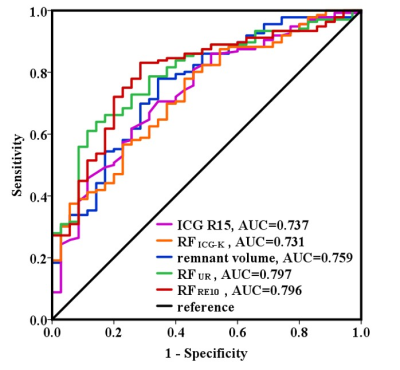
35 out of the 171 patients were classified as PHLF according to the ISGLS definition. Fig. 1 shows the example UR map and RE10 map of one patient (male, age 43) with HCC (pointed by the red arrow). The non-uniform liver function distribution can be observed in the remnant region (red contour). The patients with PHLF showed significantly lower RFICG-K, remnant volume, RFUR, RFRE10, and higher ICG R15 than the patients without PHLF (Table 1). The ROC curves of the ICG R15, RFICG-K, remnant volume, RFUR and RFRE10 are shown in Fig. 2. Imaging remnant liver function parameters (RFUR and RFRE10) have larger AUC than remnant volume and ICG based liver function parameters (ICG R15 and RFICG-K). The best cut-off value was appeared on RFRE10 (327.4ml) with a sensitivity of 83.1% and a specificity of 71.4%. The RFUR showed statistically significant correlation with the postoperative blood serum G, Alb, PA, TBIL and INR (Table 2).Discussion and Conclusion
For the first time, this study demonstrated that the remnant liver function parameters (RFUR and RFRE10) preoperatively estimated from a clinical available Gd-EOB-DTPA-enhanced imaging protocol were better than liver volume or ICG based liver function parameter in PHLF prediction, providing a new tool for liver resection planning and decision making.Acknowledgements
No acknowledgement found.References
1. World Health Organization. World Cancer Report 2014. Lyon, France: World Health Organization, 2014.
2. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40(3):225-35.
3. Greco E, Nanji S, Bromberg IL, Shah S, Wei AC, Moulton C-A, et al. Predictors of peri-opertative morbidity and liver dysfunction after hepatic resection in patients with chronic liver disease. HPB : The Official Journal of the International Hepato Pancreato Biliary Association. 2011;13(8):559-65.
4. Yang T, Zhang J, Lu JH, Yang GS, Wu MC, Yu WF. Risk factors influencing postoperative outcomes of major hepatic resection of hepatocellular carcinoma for patients with underlying liver diseases. World J Surg. 2011;35(9):2073-82.
5. Seyama Y, Kokudo N. Assessment of liver function for safe hepatic resection. Hepatol Res. 2009;39(2):107-16.
6. Imamura H, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. Journal of hepato-biliary-pancreatic surgery. 2005;12(1):16-22.
7. Makuuchi M TT, Gunven P, et al. Indication for hepatectomy in patients with hepatocellular carcinoma and cirrhosis. Shindan to Chiryo. 1986;74:1225-30.
8. Kwon AH, Ha-Kawa SK, Uetsuji S, Inoue T, Matsui Y, Kamiyama Y. Preoperative determination of the surgical procedure for hepatectomy using technetium-99m-galactosyl human serum albumin (99mTc-GSA) liver scintigraphy. Hepatology (Baltimore, Md). 1997;25(2):426-9.
9. Mao Y, Du S, Ba J, Li F, Yang H, Lu X, et al. Using Dynamic 99mTc-GSA SPECT/CT Fusion Images for Hepatectomy Planning and Postoperative Liver Failure Prediction. Annals of Surgical Oncology. 2015;22(4):1301-7.
10. Itoh S, Yoshizumi T, Shirabe K, Kimura K, Okabe H, Harimoto N, et al. Functional remnant liver assessment predicts liver-related morbidity after hepatic resection in patients with hepatocellular carcinoma. Hepatol Res. 2017;47(5):398-404.
11. Yoon JH, Choi JI, Jeong YY, Schenk A, Chen L, Laue H, et al. Pre-treatment estimation of future remnant liver function using gadoxetic acid MRI in patients with HCC. Journal of hepatology. 2016;65(6):1155-62.
12. Saito K, Ledsam J, Sourbron S, Otaka J, Araki Y, Akata S, et al. Assessing liver function using dynamic Gd-EOB-DTPA-enhanced MRI with a standard 5-phase imaging protocol. J Magn Reson Imaging. 2013;37(5):1109-14.
13. Sato Y, Matsushima S, Inaba Y, Sano T, Yamaura H, Kato M, et al. Preoperative estimation of future remnant liver function following portal vein embolization using relative enhancement on gadoxetic acid disodium-enhanced magnetic resonance imaging. Korean journal of radiology. 2015;16(3):523-30.
14. Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, et al. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-24.
Figures