0230
Hippocampal-subfield Specific Connectivity Alterations in Major Depressive Disorder Patients at 7 Tesla1Translational and Molecular Imaging Institute, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 3Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Synopsis
This study quantifies connectomic changes that occur within hippocampal subfields in patients with major depressive disorder (MDD). Using ultra-high field MRI (7 Tesla), we performed subfield-specific tractography in 6 MDD patients and 9 healthy controls. The degree of the hippocampal fissure was bilaterally increased in MDD patients compared to controls. Edgewise analyses revealed that CA3-vPFC, CA3-dlPFC, and CA1-vPFC edges were significantly increased in MDD patients compared to controls within the right hemisphere. These in vivo findings indicate that MDD patients display increased connectivity within certain hippocampal subfields, and between subfields the prefrontal cortex (PFC).
Introduction
Major depressive disorder (MDD) is a mood disorder with a lifetime prevalence of approximately 20% in the US [1]. Hallmark symptoms of MDD include, anxiety, difficulty concentrating, and persistent feelings of sadness and apathy that are not attributable to any apparent external causes [1]. Previous studies have shown volumetric changes in the hippocampal subfields of patients with MDD and other stress-related psychiatric disorders [2,3]. Additionally, alterations in fronto-limbic circuits play a critical role in motivation and affect have been linked to MDD [4]. However, connectivity of hippocampal subfields has not been investigated in patients with MDD. Probing fibers that originate in the hippocampal subfields and project to regions of the prefrontal cortex (PFC) could be important in understanding how structural connectivity may underpin functional changes in MDD. In this study we performed subfield-specific tractography and aimed to quantify alterations in subfield-PFC structural connectivity in MDD patients. We hypothesized that aberrant connectivity of hippocampal subfields will be observed in patients with MDD.Methods
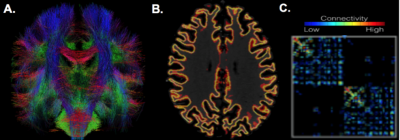
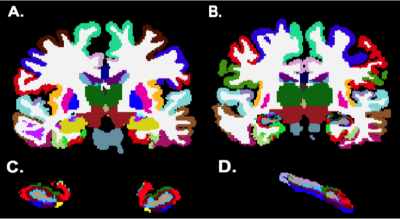
Six MDD patients and 9 healthy controls were scanned using a 7T whole body scanner (Siemens Magnetom) under an approved IRB protocol. The MRI protocol consisting of a T1-weighted MP2RAGE sequence (0.7 mm isotropic resolution) and high-angular-resolved diffusion-weighted dMRI (1.05 mm isotropic resolution, 68 directions). Diffusion-weighted images were corrected for distortions and registered to T1-weighted images. Cortical, subcortical, and hippocampal subfield segmentations were performed using FreeSurfer software [5] version 6.0. Whole brain tractography (Figure 1A) was performed using spherical deconvolution and the iFOD2 [6] algorithm and SIFT in MRTRIX3 to obtain 10,000,000 fibers. Structural connectivity matrices were calculated by counting tracks connecting each region in the FreeSurfer segmentation (Figure 2). The segmentation was used to create a grey-white matter mask that was used to seed the anatomically constrained tractography (Figure 1B), which was performed using the iFOD2, using the tckgen function from MRtrix3. Connectivity matrices were formed using the tck2connectome function from MRtrix3 (Figure 1C). A custom lookup table and parcellation image incorporating the hippocampal subfield segmentations into the wholebrain segmentation (Figure 2B) were created using the 3dcalc function from AFNI. Degrees of the hippocampal subfields were determined by calculating the total number of streamlines between the subfield and the rest of the brain in the structural matrix. A two-tailed t-test was perfomed between subfield degrees in MDD patients and controls. The streamlines of the edges connecting the hippocampal subfields to 5 regions in the PFC (ventral prefrontal cortex [vPFC], rostral middle frontal cortex [RMFC], superior frontal cortex [SFC], dorsolateral prefrontal cortex [dlPFC], and anterior cingulate cortex [ACC]) were calculated and compared between MDD patients and healthy controls using a two-tailed t-test.Results
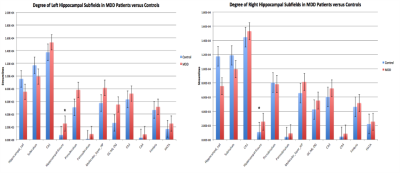
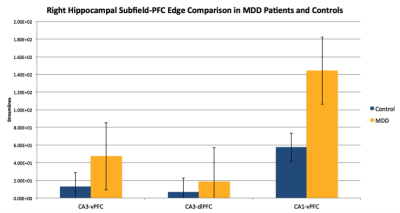
The degree analysis revealed that the streamlines going into the left hippocampal fissure were approaching significance for greater in MDD patients (Mean = 1550 $$$\pm$$$ 1110) compared to healthy controls (Mean = 736 $$$\pm$$$ 397), p = 0.06. The degree of the right hippocampal fissure was also increased in MDD patients (Mean = 2530 $$$\pm$$$ 1740) compared to controls (Mean = 1070 $$$\pm$$$ 544) , p = 0.03 (Figure 3). The edgewise analysis of tracts from the hippocampal subfields to regions of the PFC showed that within the right hemisphere, the CA3-vPFC, CA3-dlPFC, and CA1-vPFC edges were significantly greater in MDD patients than in controls, p < 0.01, p = 0.05, p =0.05, respectively (Figure 4).Discussion
This study is the first to perform tractography on hippocampal subfields in a discrete manner to quantify subfield-specific alterations in connectivity in MDD patients. We found increased degree of connectivity bilaterally in the hippocampal fissures of MDD patients. Interestingly, increased hippocampal fissure volume has been linked to anxiety-depression symptoms of certain psychiatric diseases [7]. Another study has linked elevated cortisol levels in MDD patients to increased hippocampal fissure volume [8]. These findings may help explain the increased hippocampal fissure connectivity in MDD patients. We also identify 3 subfield-PFC edges that appear to be increased in MDD patients compared with controls. Increased fronto-limbic connectivity in MDD patients has been found in previous studies and has been linked to emotional dysregulation and cognitive impairments, however this is the first study to find increased subfield-PFC connectivity [9]. Future work will include correlation analyses to determine whether connectomic changes in certain subfields are linked to specific symptoms of the disease. Additionally, a larger sample size and more robust statistical analyses will be used to validate the preliminary findings in this study and correct for multiple comparisons.Acknowledgements
Rafael O’Halloran
R01 MH109544
NARSAD Young Investigator Grant
Icahn School of Medicine Capital Campaign
Translational and Molecular Imaging Institute
References
1. Furman, Daniella J., J. Paul Hamilton, and Ian H. Gotlib. Frontostriatal functional connectivity in major depressive disorder. Biology of Mood & Anxiety Disorders, 1.1 (2011): 11.
2. Huang, Y., Coupland, N. J., Lebel, R. M., Carter, R., Seres, P., Wilman, A. H., & Malykhin, N. V. (2013). Structural Changes in Hippocampal Subfields in Major Depressive Disorder: A High-Field Magnetic Resonance Imaging Study. Biological Psychiatry, 74(1), 62-68.
3. Bremner, JD., Randall, P., Scott, TM., Bronen, RA., Seibyl, JP., Southwick, SM., Delaney, RC., McCarthy, G., Charney, DS., Innis, RB. MRI-based measurement of hippocampal volume in patients with combat- related posttraumatic stress disorder. (1995). American Journal of Psychiatry, 152(7), 973-981.
4. Johansen-Berg, H., Gutman, D. A., Behrens, T. E., Matthews, P. M., Rushworth, M. F., Katz, E., Mayberg, H. S. (2007). Anatomical Connectivity of the Subgenual Cingulate Region Targeted with Deep Brain Stimulation for Treatment-Resistant Depression. Cerebral Cortex,18(6), 1374-1383.
5. Iglesias, J.E., Augustinack, J.C., Nguyen, K., Player, C.M., Player, A., Wright, M., Roy, N., Frosch, M.P., McKee, A.C., Wald, L.L., Fischl, B., and Van Leemput, K. A computational atlas of the hippocampal formation using ex vivo, ultra-high resolution MRI: Application to adaptive segmentation of in vivo MRI. Neuroimage, 115, July 2015, 117-137.
6. Tournier J, Calamante F, Connelly A. Improved probabilistic streamlines tractography by 2nd order integration over fibre orientation distributions. In: Proc. 18th Annual Meeting of the Intl. Soc. Mag. Reson. Med. ISMRM, In: Proc. 18th Annual Meeting of the Intl. Soc. Mag. Reson. Med, 2010. p. 1670.
7. Marneros, Andreas, and Hagop S. Akiskal. The overlap of affective and schizophrenic spectra. Cambridge Univ. Press, 2009.
8. Doolin, K., Frodl, T., O’Keane, V. Relationship between hippocampal subfield volumes and cortisol awakening response parameters in major depressive disorder. Programme of the 30th ECNP Congress - Paris 2017.
9. Fang, P., Zeng, L., Shen, H., Wang, L., Li, B., Liu, L.,Hu, D. (2012). Increased Cortical-Limbic Anatomical Network Connectivity in Major Depression Revealed by Diffusion Tensor Imaging. PLoS ONE, 7(9).
Figures