0227
7T MRS in First Episode Psychosis: Neurotransmitter Deficits and Neuronal Impairment1Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2Kennedy Krieger Institute, Baltimore, MD, United States, 3Department of Psychiatry, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
The results of a 7T MRS study of a large cohort of patients with a first episode of psychosis (FEP) and healthy control subjects (HC) are reported. In patients with FEP, significant reductions in the neurotransmitters glutamate and GABA were found in selected gray matter regions, and the neuromodulator NAAG was reduced in white matter. Other metabolic abnormalities were reduced NAA (suggesting neuroaxonal damage) and glutathione. Metabolite differences were also found to depend on diagnosis. This study suggests that 7T MRS is a useful modality for understanding the molecular pathophysiology of psychosis.
Purpose
Methods
Result
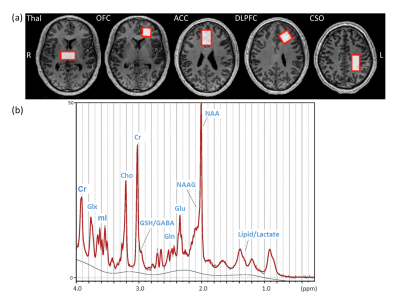
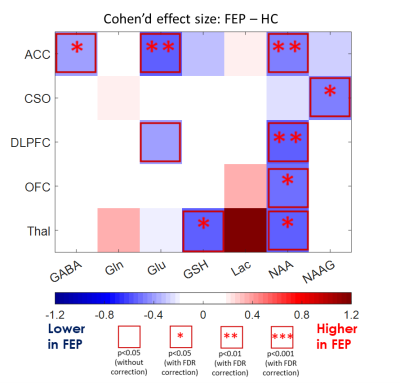
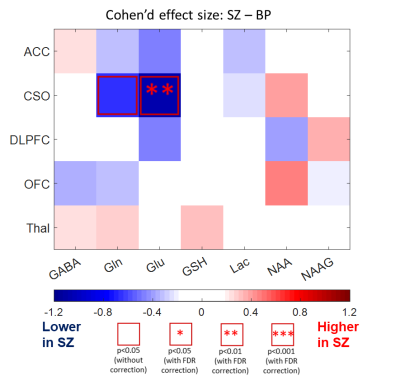
Figure 1a shows T1W images and voxel locations in a healthy control subject. A representative spectrum from the CSO with the LCModel fit is shown in Figure 1b. The concentrations of GABA and Glu in ACC, NAAG in CSO, NAA in ACC, DLPFC, OFC and Thal, as well as GSH in Thal were significantly decreased in FEP patients compared to HC (Figure 2). When comparing FEP metabolite concentrations between different diagnosis sub-groups, Glu was significantly lower in the CSO in SZ sub-group compared to BP (Figure 3).Discussion
Reduced
GABA and Glu in cortical regions, especially in the ACC, suggests involvement
of the glutamatergic and GABAergic neurotransmitter systems in FEP (3, 4). NAAG is most abundant in WM,
and the CSO voxel (~ 90% of WM) showed reduced levels of NAAG in FEP, again
suggesting that the glutamatergic system is involved. The widespread decrease
in NAA in FEP in multiple brain regions suggests that, even in the early stage
of psychosis, there was already MRS detectable neuronal loss or damage while no
significant tissue loss was detected in the T1W anatomical images (data
not shown). In addition, the reduction of the GSH might be the indication of oxidative
stress in FEP (5). The finding of reduced Glu in CSO in patients with diagnosis of SZ
(compared to BP) is interesting in that it implies differential involvement of
glutamatergic metabolism in these two diseases. In addition, prediction of
disease type may allow for early effective treatment (6).Conclusion
Acknowledgements
The authors acknowledge Mitsubishi-Tanabe Pharma. Co. Ltd. for the funding support.References
1. Merritt K, Egerton A, Kempton MJ, Taylor MJ, McGuire PK. Nature of Glutamate Alterations in Schizophrenia: A Meta-analysis of Proton Magnetic Resonance Spectroscopy Studies. JAMA Psychiatry. 2016;73(7):665-74.
2. Thakkar KN, Rosler L, Wijnen JP, Boer VO, Klomp DW, Cahn W, et al. 7T Proton Magnetic Resonance Spectroscopy of Gamma-Aminobutyric Acid, Glutamate, and Glutamine Reveals Altered Concentrations in Patients With Schizophrenia and Healthy Siblings. Biol Psychiatry. 2017;81(6):525-35.
3. Stone JM, Morrison PD, Pilowsky LS. Glutamate and dopamine dysregulation in schizophrenia--a synthesis and selective review. J Psychopharmacol. 2007;21(4):440-52.
4. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312-24.
5. Fendri C, Mechri A, Khiari G, Othman A, Kerkeni A, Gaha L. Oxidative stress involvement in schizophrenia pathophysiology: a review. L'Encephale. 2006;32(2 Pt 1):244-52.
6. Patel KR, Cherian J, Gohil K, Atkinson D. Schizophrenia: overview and treatment options. Pharmacy and Therapeutics. 2014;39(9):638.
Figures