0225
N-acetyl-cysteine supplementation improves functional connectivity in the cingulate cortex in early psychosis1Radiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 2Dutch connectome lab, University Medical Center (UMC), Utrecht, Netherlands, 3Service of General Psychiatry and Center for Psychiatric Neuroscience, Department of Psychiatry, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 4Department of psychiatry, Lausanne University Hospital (CHUV), Lausanne, Switzerland
Synopsis
Schizophrenia implies different alterations in prefrontal cortex and in particular, a disrupted connectivity in this brain area and a redox dysregulation. In a previous analysis, glutathione levels (main antioxidant and redox regulator) correlated with functional connectivity within the cingulate cortex. In this study, we investigate the effect of N-acetyl-cysteine (NAC) (precursor of glutathione) supplementation on functional connectivity in early psychosis patients. The results show an increased functional connectivity strength and betweenness centrality in these regions, suggesting that cingulate cortex functional connectivity could be a biomarker for NAC treatment efficacy
Introduction
Early intervention in schizophrenia is essential to improve outcome. New treatments based on mechanisms in schizophrenia are needed and increasing evidence suggests that oxidative stress is involved on the disease pathophysiology [1]. Indeed, estimation of the level of gluthatione (GSH), main actor of the brain redox dysregulation, using Magnetic Resonance Spectroscopy (MRS), has revealed a decreased GSH levels in early psychosis patients (EPP). Moreover, Magnetic Resonance Imaging (MRI) techniques have highlighted structural and functional connectivity (FC) disruptions along the cingulum bundle for schizophrenic patients [2,3]. N-acetyl-Cysteine (NAC) is an antioxidant and precursor of GSH, almost devoid of side effects. In chronic patients, add-on of NAC improved negative symptoms and reduced side effects of antipsychotics [4]. In a recent double-blind randomized placebo-controlled trial, early psychosis patients received NAC supplementation (2700 mg/day, 6 months) [5]. There were significant improvements in NAC treated patients on cognition and positive symptoms. Whether NAC treatment leads to improvement in FC in EPP is unknown. Given these connectivity disruptions and given that GSH levels correlated with FC within the cingulate cortex in EPP [6], we investigate the effect of NAC supplementation during 6 months in EPP on cingulum cortex FC.Methods
Twenty EPP which constitutes a subgroup of the double-blind randomized placebo-controlled trial by [7] were scanned with MRI imaging. T1-weighted volumes and resting-state fMRI recording were acquired on a Siemens-3T scanner in EPP who received either NAC (n=9) or placebo (n=11) as add-on treatment at baseline (T1) and follow-up (T2) after 6 months. A control group (CTRL) (n=93) was scanned in the same conditions. T1-weighted sequence had 1 mm in-plane resolution, 1.2 mm slice thickness, with TR/TE/TI 2300/2.98/900 ms; fMRI gradient-echo-EPI sequence had 3.3x3.3x3.3 mm^3 resolution, with TR/TE 1920/30 ms. T1-weighted volumes were parcellated into 68 regions using Freesurfer. Data were processed with CMTK [8] according to a state-of-the-art pipeline, including motion correction, nuisance signals regression, scrubbing, band-pass filtering. Time series were averaged over the Freesurfer cortical regions and Pearson’s correlation was used as FC measure. We investigated FC changes occurring in the cingular cortices (Fig1) between T1 and T2 for NAC and placebo EPP and between EPP and CTRL by looking at the FC strength of these regions. Moreover, we assessed global efficiency and edge betweenness centrality (BC) for the whole brain network.Results
FC between caudal and isthmus cingulate cortices increases significantly after 6 months for patients who received NAC supplementation (p=0.01, ranksum test) (Fig2A), but not for patients who received the placebo. FC of these regions was also higher in patients after 6 months of NAC supplementation (but not in patients after receiving the placebo, or in patients at baseline) (Fig2B). Regarding the local network measures, BC differences are observed for the same regions. Indeed, edge BC increases between caudal and isthmus cingulate regions after NAC supplementation compared to placebo (p=0.015) (Fig3A). At the nodal level, the increase of BC can be mainly ascribed to the isthmus cingulate cortex (p=0.01) (Fig3B). No significant global network measures differences were found between T1 and T2 between placebo and NAC patients.Discussion
NAC supplementation has an impact on FC between caudal and isthmus cingulate regions in our dataset. NAC increases FC strength between these two regions. This strength is significantly higher than for matched control subjects. NAC has a stimulating effect on FC in regions along the cingulum bundle. This FC change could be partially explained by the changes in BC in resting-state functional networks for these regions. The edge BC of the caudal and isthmus cingulate connection is increased for NAC subjects: a highest number of shortest paths go through this connection after NAC supplementation. It shows a growing importance of this edge in connecting the frontal and posterior regions of the brain network. The isthmus cingulate cortex appears to be particularly affected by NAC and could be a potential biomarker of functional connectivity to assess treatment response to NAC. A higher number of subjects would allow to confirming the robustness of these findings. This analysis is limited to the regions along the cingulum bundle but further analysis will be extended to the whole brain.Conclusion
This is a first study assessing the impact of NAC treatment on FC in a sample of EPP. After 6 months, NAC improves FC in caudal anterior and isthmus cingulate cortices. This FC increase is characterized by a higher BC in these brain areas, improving the connection between the frontal and posterior regions along the cingulum bundle. These results suggest that FC along the cingulum may be a biomarker for NAC treatment response.Acknowledgements
No acknowledgement found.References
[1] Oxidative stress-driven parvalbumin interneuron impairment as a common mechanism in models of schizophrenia. Steullet P, Cabungcal JH, Coyle J, Didriksen M, Gill K, Grace AA, Hensch TK, LaMantia AS, Lindemann L, Maynard T, Meyer U, Morishita H, O’Donnell P, Puhl M, Cuenod M, Do KQ. Molecular Psychiatry, 22 (7), 936-943 (2017)
[2] Altered functional and anatomical connectivity in schizophrenia. J. Camchong, A. W. MacDonald III, C. Bell, B. A. Mueller, K. O. Lim. Schizophrenia Bulletin (vol37 n°3 pp. 640-650) (2011).
[3] Localized abnormalities in the cingulum bundle in patients with schizophrenia: A Diffusion Tensor tractography study. T. J. Whitford, S.W. Lee, J. S. Oh, R. de Luis-Garcia, P. Savadjiev, J. L. Alvarado, C.-F. Westin, M. Niznikiewicz, P. G. Nestor, R. W. McCarley, M. Kubicki, M. E. Shenton. NeuroImage:Clinical 5 93-99 (2014).
[4] N-acetyl-cysteine as a glutathion precursor for schizophrenia – A double blind, randomized, placebo-controlled trial. Berk M., Copolov D., Dean O., Lu K., Jeavons S., Schapkaitz I., Anderson-Hunt M., Judd F., Katz F., Katz P, Ording-Jespersen S., Little J., Conus P., Cuenod M., Do K., Bush A. Biological Psychiatry vol64, issue 5, 361-368 (2008)
[5] N-Acetyl-Cysteine in a double-blind randomized placebo-controlled trial: Towards biomarker guided treatment in early psychosis. Conus P, Fournier M, Xin L, Baumann PS, Ferrari C, Cousins A, Alameda L, Gholam-Rezaee M, Golay P, Jenni R, Woo TW, Keshavan MS, Eap CB, Wojcik J, Cuenod M, Buclin T, Gruetter R, Seidman LJ, Do KQ. Schizophrenia Bulletin, doi:10.1093/schbul/sbx093, in press
[6] Glutathione deficit impairs myelin maturation: relevance for white matter integrity in schizophrenia patients. A. Monin, P.S. Baumann, A. Griffa, L. Xin, R. Mekle, M. Fournier, C. Butticaz, M. Klaey, J.H. Cabungcal, P. Steullet, C. Ferrari, M. Cuenod, R. Gruetter, J.P. Thiran, P. Hagmann, P. Conus, K. Q. Do. Molecular Psychiatry 20, 827-838 (2015)
[7] N-acetyl-cysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis. P. Conus, L. J. Seidman, M. Fournier, L, Xin, M. Cleusix, P. S. Baumann, C. Ferrari, A. Cousins, L. Alameda, M. Gholam-Rezaee, P. Golay, R. Jenni, T.-U. Wilson Woo, M. S. Keshavan, C. B. Eap, J. Wojcik, M. Cuenod, T. Buclin, R. Gruetter, K. Q. Do. Schizophrenia Bulletin (2017)
[8] The Connectome Mapper: An open-source processing pipeline to map connectomes with MRI. Daducci A., Gerhard S., Griffa A., Lemkaddem A., Cammoun L., Gigandet X., Meuli R., Hagmann P., Thiran JP. PlosOne
Figures
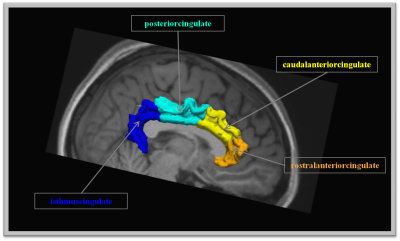
Regions along the cingulum bundle
Regions connected by the cingulum bundle and selected for the study.
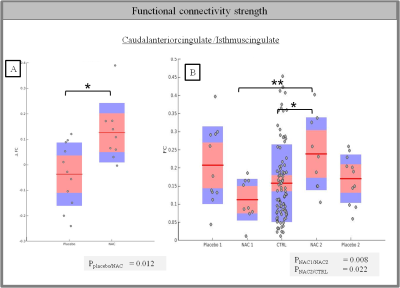
Functional connectivity changes in the cingulate cortex after NAC supplementation
The central mark is the mean and the edges of the box are the 25th and 75th percentiles. A rank sum test is used to calculate the p-value. (* p<0.05 , ** p<0.01)
A) Difference of functional connectivity strength between scan 1 and scan 2 for NAC and placebo patients in caudal anterior and isthmus cingulate cortices
B) Average functional connectivity for the different groups of subjects
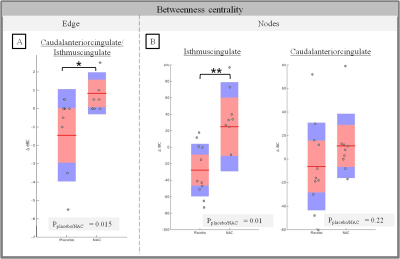
Centrality changes in the cingulate cortex after NAC supplementation
The central mark is the mean and the edges of the box are the 25th and 75th percentiles. A rank sum test is used to calculate the p-value. (* p<0.05 , ** p<0.01)
A) Difference of edge betweenness centrality between scan 1 and scan 2 for NAC and placebo patients for caudal anterior/isthmus cingulate edges
B) Difference of node betweenness centrality between scan 1 and scan 2 for NAC and placebo