0224
Impaired modulation of hippocampal glutamate during memory consolidation in schizophrenia: Evidence from ¹H fMRS1Psychiatry and Behavioral Neurosciences, Wayne State University School of Medicine, Detroit, MI, United States
Synopsis
Schizophrenia is one of the most debilitating, life-long mental illnesses where treatment has a limited impact in restoring real-life functions such as deficits in learning and memory. The hippocampus is particularly rich in glutamatergic neurons and altered neuroplasticity related to glutamate has been proposed as a critical mechanism mediating learning and memory in schizophrenia. Understanding glutamate-related dysfunction in schizophrenia may therefore, elucidate mechanisms underlying the illness as well as help tailor intervention strategies. Here we provide the first ever evidence of a dysfunctional modulation of hippocampal glutamate during memory encoding in schizophrenia using ¹H fMRS.
Introduction
Glutamate plays a central role in frontal-hippocampal mechanisms of learning and memory. The hippocampus is particularly rich in glutamatergic neurons and N-methyl-D-aspartate (NMDA) mediated synaptic plasticity is central for subserving learning. Unsurprisingly, altered neuroplasticity related to glutamate has been proposed as a critical mechanism mediating learning and memory deficits in schizophrenia. Understanding glutamate-related hippocampal dysfunction in schizophrenia may therefore, elucidate not only mechanisms underlying the illness, but may subsequently help in tailoring intervention strategies. However in vivo studies have largely relied on hemodynamic signals based on fMRI to assess hippocampal function (and dysfunction) in schizophrenia, or on quantitating static levels of glutamate with ¹H MRS. These signals are not informative of functional modulation of hippocampal glutamate. Here we provide the first ever application of in vivo ¹H functional MRS (fMRS) to assess dysfunctional modulation of hippocampal glutamate in schizophrenia. fMRS is a highly novel method for quantitating functional neurochemistry and can provide direct evidence of normal and impaired hippocampal function (Stanley et al., 2017).Methods
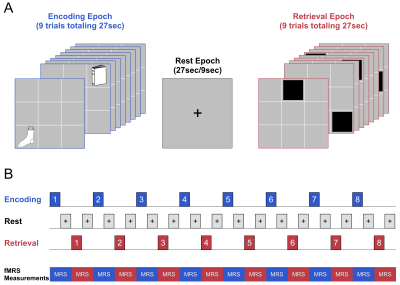
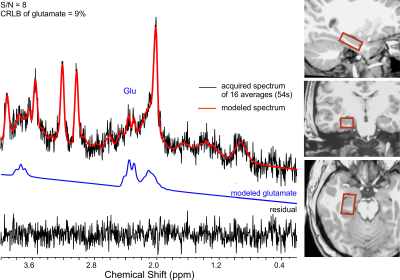
Fifteen early course DSM-V schizophrenia patients (10M + 5F; 25.9±3.7yrs) and 13 healthy, young adults (8M + 5F; 25.8±3.9yrs) participated in the ¹H fMRS study at 3T. The associative learning and memory previously assessed in schizophrenia using fMRI (Wadehra et al., 2013) involved epochs of encoding (9 unique object-location pairs) and cued-retrieval (of those associated memoranda) and interspersed with rest epochs (Stanley et al., 2017) (Figure 1). Eight encoding-retrieval cycles were employed to allow learning to asymptote. Mixed rest epochs of either 27s (64% of data) or 9s were interspersed between task blocks. A visual flashing checkerboard task was introduced prior to the onset of the learning task to provide a non-hippocampal task as a non-memory baseline condition. A total of 19 consecutive single-voxel, short-TE ¹H spectra of 16 averages each were acquired from the right head/body of the hippocampus (voxel:1.7x3.0x1.2cm or 6.12 cm3, PRESS, TE=23ms). The resulting ¹H spectra from the 8 encoding/rest epochs and 8 retrieval/rest epochs as well the 3 ¹H spectra from the baseline condition were quantified using LCModel (Figure 2). Performance on task was estimated by modeling the behavioral data (expressed as a percent correct by epoch) using the three-parameter Gompertz function (learning rate, asymptote and inflection point). The statistical analyses assessed changes in glutamate across task conditions (encoding, retrieval) relative to baseline for each group as well as a group-by-task condition interaction for glutamate using the repeated measure GEE approach with task (encoding, retrieval) and time (epoch number) as within subject factors, and age and sex as covariates.Results
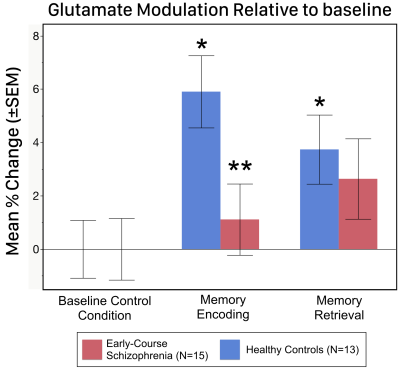
Regarding performance, both groups showed negatively accelerated learning with no statistical group differences on the learning rate, asymptote or inflection point (all p>.20). Independent of time, the task condition for glutamate was significant in controls (Χ2= 7.70; p=0.021) with post-hoc tests demonstrating a significant increase in hippocampal glutamate during both encoding (5.9%; p<.0001) and retrieval (3.7%, p=.028) compared to baseline (Figure 3). In contrast, there were no significant differences in glutamate across conditions in schizophrenia patients. Additionally, the group-by-task interaction was not significant (Χ2= 1.45; p=.23); however, post-hoc analyses indicated a significantly higher glutamate modulation during encoding in controls compared to schizophrenia (p=0.025), while glutamate modulation during retrieval was not significant between groups (p=.64).Discussion
The results indicate that even as schizophrenia subjects were engaged in the task, modulation of hippocampal glutamate was absent during encoding and retrieval. These results provide the first assessment, and evidence of impaired functional neurochemistry of the hippocampus in schizophrenia. In healthy controls, the increased levels of hippocampal glutamate observed during encoding and retrieval may be driven by the influx of oxidative metabolism related to increased neuronal activity (Schaller et al., 2014), but assessed independently of the confounds of hemodynamics. By inference, therefore, impaired glutamate modulation in schizophrenia may reflect a lack of neuronal engagement during learning. Though further studies are warranted (and are ongoing), our results provide the first direct evidence of a hippocampal dysfunction related to glutamatergic neurotransmission during processes of memory consolidation in schizophrenia.Acknowledgements
This study was supported by NIMH under award number R01 MH111177 (JAS and VAD) and by the Lycaki-Young Funds from the State of Michigan.References
Schaller, B., Xin, L., O'Brien, K., Magill, A.W., Gruetter, R., 2014. Are glutamate and lactate increases ubiquitous to physiological activation? A (1)H functional MR spectroscopy study during motor activation in human brain at 7Tesla. NeuroImage 93 Pt 1, 138-145.
Stanley, J.A., Burgess, A., Khatib, D., Ramaseshan, K., Arshad, M., Wu, H., Diwadkar, V.A., 2017. Functional dynamics of hippocampal glutamate during associative learning assessed with in vivo (1)H functional magnetic resonance spectroscopy. NeuroImage 153, 189-197.
Wadehra, S., Pruitt, P., Murphy, E.R., Diwadkar, V.A., 2013. Network dysfunction during associative learning in schizophrenia: Increased activation, but decreased connectivity: an fMRI study. Schizophr Res 148, 38-49.
Figures