0221
MEG-Navigators for Motion Detection and Quality Assurance in MR Elastography1Institute for Biomedical Engineering, University and ETH Zurich, Zurich, Switzerland
Synopsis
We propose to use the motion encoding gradients (MEGs) of a conventional 3D GRE-MRE sequence as efficient 1D projection navigators with only minor changes to the sequence timing. We show that MEG-NAVs can be used to detect breathing motion, flexing of the thigh muscle, as well as changes in magnitude and phase of the MRE transducer. The additional MEG-NAV data can be used to check breath-hold compliance in conventional GRE-MRE liver exams as well as to ensure optimal transducer operation.
Introduction
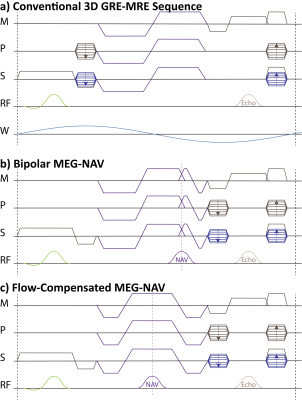
In MR Elastography (MRE), motion encoding gradients (MEGs) are used to sensitize a phase-contrast sequence to the periodic motion induced by an external wave generator (Figure 1a)1. While MRE of the liver is typically performed in a breath hold2, no information about actual breath-hold compliance as well as transducer operation is collected other than the presence of imaging artifacts in the final result. In this work, we propose to slightly modify a conventional 3D GRE-MRE sequence allowing to use flow-compensated and bipolar MEGs as 1D projection navigators with little to no change in sequence timing and encoding efficiency. These “MEG-NAVs” can be used to continuously track breathing motion, breath-hold compliance, flexing of e.g. the thigh muscles, as well as optimal transducer operation.Theory
It is proposed to shift phase encoding and measurement pre-phasing gradients after the MEG, while the slice rewinding gradient is played out before the MEG. In this way, the MEGs start and end in the k-space center and can be exploited as projection navigators. If flow-compensated MEGs are used, the navigator FID can be read out in the center of the 1-2-1-MEG as shown in Figure 1b without any further alternations in the sequence. Bipolar MEGs only allow for the acquisition of a center-out radial projection. By appending a short, reversed MEG waveform (Figure 1c), the full projection can be acquired, while encoding efficiency is only slightly reduced.
The acquired projections of the excited volume depend on the employed encoding scheme. Hadamard encoding is predestined, as it consists of four projections along the diagonals of a regular cube while providing the highest encoding efficiency of four-point schemes for motion sensitization3. By varying the encoding direction in either sequential (Figure 2a) or interleaved (Figure 2b) fashion, a projection can be acquired for each wave phase, encoding direction and k-line.
Since the acquired MEG-NAVs are also phase-locked to the wave generation and played either during (flow-compensated) or after motion sensitization (bipolar), they not only allow for the estimation of conventional motion data, e.g. breathing, but also allow for the global tracking of the induced wave field. The magnitude of the MEG-NAV is modulated by intra-voxel phase dispersion (IVPD) originating from the underlying motion field and the strong motion-sensitization in MRE, which is further increased due to the large projection volume4,5. By correlating the projection magnitudes of MEG-NAVs at different time-points, changes in amplitude and phase of the wave field can be detected.
Methods
The proposed MEG-NAVs were implemented in a conventional, fractional 3D GRE-MRE sequence on a Philips Achieva 1.5T and a Philips Ingenia 3T scanner. Data for in-vivo breathing motion extraction was acquired as a proof-of-concept without wave actuation at 1.5T, using a fictional 30Hz actuation, sequential acquisition and flow-compensated MEGs with centered readout. MEG-NAV acquisition in the thigh was performed on the 3T system using 35Hz electro-magnetic actuation, unbalanced four-point encoding, and bipolar MEGs of 255Hz. Phantom data was also acquired at 3T, with 60Hz actuation, Hadamard encoding and bipolar MEGs of 170Hz.
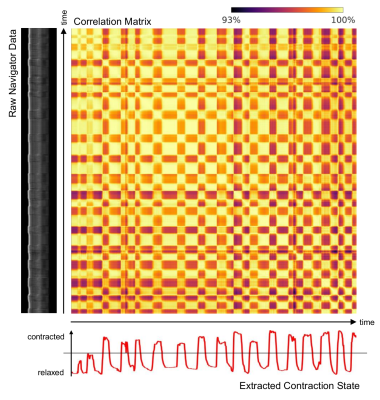
The processing of the navigator data is described in the figure captions. All analyses depend on pairwise correlation6 and subsequent singular-value decomposition (SVD) and are based on the second eigenvector (EV). All estimates were smoothed using a moving average filter of kernel size 32, which is the product of encoding directions and acquired phase offsets per k-line.
Results and Discussion
In Figure 3, breathing motion estimation is demonstrated using MEG-NAVs. The resulting breathing estimate is in very good agreement with the respiratory bellow signal. Since Hadamard encoding acquires four different projections, self-gating using Hadamard MEG-NAVs is more robust as e.g. 1D projection navigators from pseudo-radial/-spiral or stack-of-star acquisitions that rely on a single direction7,8.
In Figure 4, contraction state estimation for the thigh is demonstrated using a single sagittal projection. The volunteer was instructed to alternate between contraction and relaxation of the thigh, which was picked up by the MEG-NAVs.
In Figure 5, phantom results are shown for an ultrasound gel phantom, where the amplitude and phase of the transducer was changed during MRE acquisition, which was reconstructed using the MEG-NAV signal. Normally, changes in the wavefield go unnoticed and might only manifest in image artifacts. Temporal correlation of the MEG-NAV signal allows for the detection of amplitude and phase changes in the induced wave field.
Conclusions
We have proposed the use of motion encoding gradients in MRE as projection navigators and have demonstrated their ability to detect breathing motion, contraction of muscles, and changes in amplitude and phase of the transducer. MEG-NAVs can be used for self-gating and quality assurance in MRE, e.g. to check breath-hold compliance in MRE liver exams and proper operation of the transducer.Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039.References
1. Muthupillai R, Lomas D, Rossman P, Greenleaf J, Manduca A, Ehman R. Magnetic resonance elastography by direct visualization of propagating acoustic strain waves. Science (80). 1995;269(5232):1854-1857. doi:10.1126/science.7569924.
2. Garteiser P, Sahebjavaher RS, Ter Beek LC, et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR Biomed. 2013;26(10):1326-1335. doi:10.1002/nbm.2958.
3. Guenthner C, Runge JH, Sinkus R, Kozerke S. Hadamard Encoding for Magnetic Resonance Elastography. In: Intl. Soc. Mag. Reson. Med. 25. Honolulu; 2017:1378.
4. Glaser KJ, Felmlee JP, Manduca A, Ehman RL. Shear Stiffness Estimation Using Intravoxel Phase Dispersion in Magnetic Resonance Elastography. Magn Reson Med. 2003;50(6):1256-1265. doi:10.1002/mrm.10641.
5. Yin Z, Kearney SP, Magin RL, Klatt D. Concurrent 3D acquisition of diffusion tensor imaging and magnetic resonance elastography displacement data (DTI-MRE): Theory and in vivo application. Magn Reson Med. 2017;77(1):273-284. doi:10.1002/mrm.26121.
6. Wundrak S, Paul J, Ulrici J, et al. A self-gating method for time-resolved imaging of nonuniform motion. Magn Reson Med. 2016;76(3):919-925. doi:10.1002/mrm.26000.
7. Uribe S, Muthurangu V, Boubertakh R, et al. Whole-heart cine MRI using real-time respiratory self-gating. Magn Reson Med. 2007;57(3):606-613. doi:10.1002/mrm.21156.
8. Feng L, Axel L, Chandarana H, Block KT, Sodickson DK, Otazo R. XD-GRASP: Golden-angle radial MRI with reconstruction of extra motion-state dimensions using compressed sensing. Magn Reson Med. 2016;75(2):775-788. doi:10.1002/mrm.25665.
9. Zhang T, Cheng JY, Chen Y, Nishimura DG, Pauly JM, Vasanawala SS. Robust self-navigated body MRI using dense coil arrays. Magn Reson Med. 2016;76(1):197-205. doi:10.1002/mrm.25858.
10. Cheng JY, Alley MT, Cunningham CH, Vasanawala SS, Pauly JM, Lustig M. Nonrigid motion correction in 3D using autofocusing withlocalized linear translations. Magn Reson Med. 2012;68(6):1785-1797. doi:10.1002/mrm.24189.
Figures