0216
Shuttered EPI Brain Imaging at 7 Tesla1Department of Radiology, Vanderbilt University Institute of Imaging Science, Vanderbilt University Medical Center, Nashville, TN, United States, 2Department of Radiology, A.A. Martinos Center for Biomedical Imaging, Harvard Medical School, Charlestown, MA, United States, 3Department of Biomedical Engineering, Vanderbilt University Institute of Imaging Science, Vanderbilt Univserity, Nashville, TN, United States
Synopsis
Achieving robust sub-millimeter resolution EPI at 7 Tesla is a challenge since conventional single shot images suffer from long echo times and severe image distortions, while multishot EPI suffers from motion- and respiration-induced shot-to-shot phase errors. Recently, Taviani et al described an ‘in-plane multiband’ or ‘shuttered EPI’ method for 3T breast diffusion imaging, in which data are acquired in each shot after exciting a set of shutters across the imaged slice and combined using a phase- and motion-insensitive reconstruction. Here we present an implementation of this approach for high-resolution 7T brain imaging, which required a new RF pulse construction.
PURPOSE
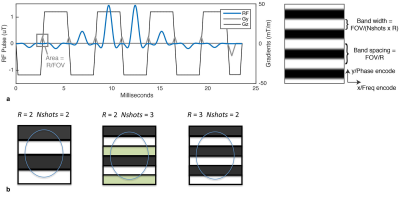
Echo Planar Imaging (EPI) is the most widely used sequence for rapid functional, diffusion and perfusion MRI at high field1. In recent years, considerable effort has been invested in increasing the resolution of EPI in order to measure brain structure and function at sub-millimeter scales2,3. While single shot EPI has remained the main sequence for achieving these targets, it suffers from long readout times that reduce signal and sensitivity in functional and diffusion MRI, and from high sensitivity to off-resonance induced distortions and T2*-induced blurring. The traditional approach to counter these effects is Multishot EPI (mshEPI)4. However, classic k-space segmented mshEPI is seldom used due to its vulnerability to motion and respiration-induced shot-to-shot phase inconsistencies5,6. The purpose of this work is therefore to introduce a mshEPI method called Shuttered EPI which can address the limitations of conventional mshEPI by imaging a set of disjoint shutters in each shot (Figure 1), which are shifted between shots to cover the entire slice. This enables images to be separately reconstructed and motion-corrected from each shot, which can be combined to obtain a full slice image. Here we present sequence design and image reconstruction for shuttered EPI at 7 Tesla.METHODS
RF Pulse Design: Figure 2 shows a two-dimensional shuttered EPI excitation pulse. It is similar to a PINS pulse for SMS imaging7, in that it exploits excitation aliasing to produce equispaced copies of the shutter pattern across the slice, to minimize its power. It is slice-selective in its frequency-encoded dimension and shutter-selective in its blipped dimension, with blip areas equal to the inverse of the shutter spacing. The shutter widths are equal to the encoded FOV of each shot, FOV/R, where R is the parallel imaging acceleration factor. For each shot, phase increments are added to the individual sub-pulses to shift the shutters spatially and cover the entire slice.
Pulse Sequence: A conventional interleaved mshEPI sequence was modified to include the two-dimensional RF pulse described above, and to apply phase shifts to its sub-pulses to shift the shutter pattern between shots. 0th-order phase corrections were performed to minimize between-shutter ghost excitations; they could be further reduced with 1st order (delay) corrections.
Image Reconstruction: After standard N/2 ghost corrections, images were reconstructed using SENSE8. SENSE coil-sensitivity maps were estimated as the ratio of individual resolved shutter images to a sum-of-squares full excitation image.
Experiment: An adult human volunteer was scanned in a 7 Tesla scanner (Philips Healthcare, Best, The Netherlands) with a single-channel transmit, 32-channel receive coil setup. A brain slice was imaged with a FOV of 202 mm and 1 x 1 x 4 mm3 resolution. The shuttered pulse was designed for 4 shots and R = 3, with slice and shutter time bandwidth products of 4, FWHM shutter width of 22.4 mm and shutter overlap of 5.56 mm. The excitation pulse was 17.5 ms long and included 12 sub-pulses. Data were acquired with 12 shots retrospectively decimated to 4 shots for R = 3 undersampled reconstruction, 17 echoes per shot and TE/TR of 22/3000 ms. A 12-shot conventional mshEPI image was also acquired and decimated to R = 3 for comparison. Compared to single-shot readouts, the total readout length was therefore reduced by a factor of 12 in each case, resulting in very little residual distortion. Breathholds were used for both scans to minimize artifacts and facilitate comparison between conventional mshEPI and shuttered EPI based on image encoding and reconstruction quality. Spectral Presaturation with Inversion Recovery (SPIR) fat suppression was used for all acquisitions.
RESULTS
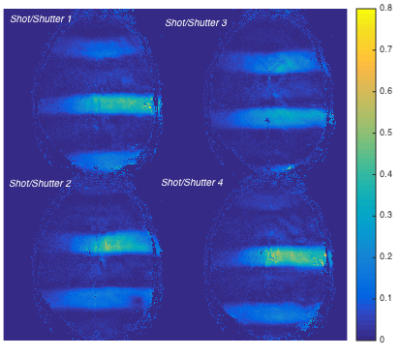
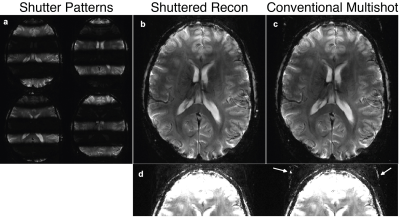
Figure 3 displays SENSE receive maps for one receiver channel. Figure 4 shows the reconstructed shuttered EPI image, along with the individual shutter images and the conventional mshEPI image. The shuttered image is nearly indistinguishable from the full excitation image. When scaled to 25% of the maximum combined signal, some residual ghosting is visible in the conventional multishot image that is reduced in the shuttered imageDISCUSSION
We have described an initial implementation of shuttered EPI at 7T, which should enable high-resolution fMRI and dMRI with low motion and physiological noise sensitivity, without the use of navigators. Our immediate next steps will be to develop motion and phase error-robust reconstructions and assess their sensitivity to motion and physiological noise. We will further combine it with simultaneous multislice imaging to increase slice coverage. We have also developed parallel transmission shutter pulses with shorter durations than the 2D pulses used here.9Acknowledgements
This work was supported by NIH R01 EB 016695.References
1. Mansfield, P. J Phys, C: Solid State Phys 10, L-55-58 (1977) .
2. Lewis LD et al, Proc Nat Acad Sci, 113(43) E66790E6685. (2016).
3. Feinberg DA et al, PLoS One, 5(12):e 15710 (2010).
4. McKinnon GC Magn Reson Med, 30, 609-616 (1993).
5. Polimeni JR et al, Magn Reson Med, 75(2), 665-679 (2016).
6. Zur Y. Magn Reson Med, 66(6), 1616-1626 (2011).
7. Norris et al Magn Reson Med 66(5):1234-1240 (2011).
8. Pruessmann KP et al, Magn Reson Med 42(5), 952-962 (1999)
9. Cao Z et al, ISMRM 2018 (Submitted)
Figures