0212
Tilted-CAIPI for Highly Accelerated Distortion-Free EPI with Point Spread Function (PSF) Encoding1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 2A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 3Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States
Synopsis
The main challenge in high-resolution EPI is the significant B0-distortion and T2*-blurring artifacts. PSF-encoded EPI has been proposed to achieve distortion- and blurring- free imaging at a cost of extremely long acquisition time, not practical for most applications. In this work, we introduce the “tilted-CAIPI” method, which can provide >20x acceleration for PSF-EPI by utilizing the concept of B0-inhomogeneity encoding and optimized sampling. With the proposed method, PSF-encoded EPI at a 1mm resolution range can be obtained using just 4-8 EPI-shots. PSF-EPI with tilted-CAIPI was demonstrated for efficient acquisition of distortion- and blurring- free T2-weighted, T2*-weighted, and diffusion-weighted images.
Introduction
Point Spread Function (PSF) mapping[1,2] encodes an additional phase-encoding (kpsf) into EPI acquisition to provide a highly reliable mapping of B0-distortion and T2*-blurring. Due to its encoding-intensive nature, which requires >100 EPI-shots, PSF-EPI is typically performed as a pre-scan, to estimate a distortion map used for post-processing correction of subsequent EPI scans. Such correction nicely removes B0-distortions, but does not remove T2*-blurring, and also lead to significant loss in resolution and anatomical details in strong susceptibility areas.
Methods have been proposed to accelerate PSF encoding by 5-8× through a combination of reduced-FOVpsf and parallel imaging along PE[3], or along PE and PSF[4]. This has recently permitted PSF-EPI to be used for imaging directly, to achieve very high-quality diffusion imaging at 7T[3]. Nonetheless, even with such accelerations, 30-40 EPI-shots are still needed to acquire a single brain imaging slice at 1mm resolution. Here, we propose the “tilted-CAIPI” scheme, which systematically optimized the PSF-PE under-sampling with B0-inhomogeneity-informed parallel imaging reconstruction to achieve >20× PSF acceleration. Tilted-CAIPI combined with SMS and partial Fourier acquisition was demonstrated for fast, distortion- and blurring-free imaging at 1mm resolution range with 4-8 shots, for whole-brain imaging in 20-30s.
Methods
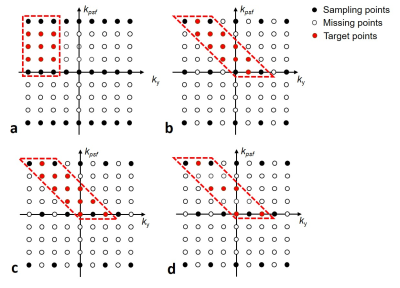
PSF-EPI acquires a 3D k-space (kx-ky-kpsf) for each slice, where ky and kpsf are both gradient-encoding along y, with each EPI-shot having an extra pre-winding blip to achieve a particular kpsf encoding step. At a certain ky, all the PSF-encoding signals are acquired at the same echo time, therefore, there is no distortion and T2* blurring along the PSF direction. In conventional accelerated PSF-EPI[4], the ky-kpsf encodings are under-sampled by RPE×RPSF and recovers using sequential PE-GRAPPA and PSF-GRAPPA reconstructions (Fig.1a). Here, RPE is used to reduce effective echo spacing (ESP) and thereby the level of distortion/blurring, which enables higher RPSF accelerations (i.e. less EPI-shots).
To achieve higher acceleration for PSF-EPI, “tilted-CAIPI” is proposed to exploit the inherent correlation of PE-PSF encoding through optimized sub-sampling and reconstruction. In PSF-EPI, kpsf encoding utilizes a Gy pre-winding blip to shift the effective phase-encoding of each EPI-shot. Therefore, data points along a -45° diagonal line in the ky-kpsf plane share the same effective y-gradient encoding, with additional encodings from B0-inhomogeneity induced phase and T2/T2* signal decay. Since the time difference are very small among neighboring points along such diagonal line (a few milliseconds), a tilted compact kernel operator in ky-kpsf across multi-coil data should be able to effectively capture the small B0-inhomogeneity induced phase and interpolate small T2* decay, to recover the under-sampled data along ky-kpsf. Thus, tilted-CAIPI approach utilizes a tilted kernel (Fig.1b), and a tilted 2D-CAIPI[5] under-sampling pattern along ky-kpsf (Fig.1c) to make full use of PE-PSF correlation and parallel imaging.
To further reduce acquisition time, tilted-CAIPI is combined with blipped-CAIPI SMS[6] and partial Fourier acquisition, where partial Fourier along PSF and PE are recovered using 3D POCS reconstruction. For kernel training, low-resolution fully-sampled PSF-EPI is acquired with matching effective ESP, where calibration scan time is reduced using i) SMS, and ii) reduced FOVpsf reconstruction (Fig.1d) which reduces the number of kernels to be trained.
Acquisitions: PSF-EPI brain data were acquired at 3T for i) T2-weighted spin-echo (SE) imaging at 1×1×3 mm3 and 0.9×0.9×1mm3, ii) Gradient-echo (GE) and spin-echo at 0.8×0.8×3 mm3, and iii) DWI at 0.8×0.8×3 mm3 and 1.2mm-isotropic resolution. For DWI data, PSF-EPI acquisition and reconstruction were modified to achieve self-navigated correction for diffusion phase corruption[8].
Results
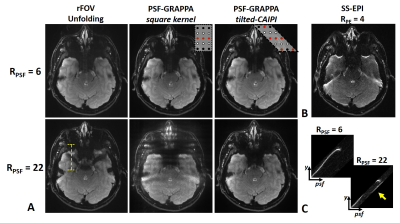
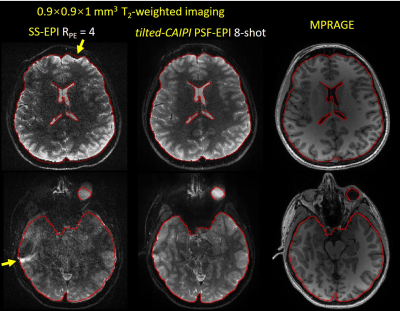
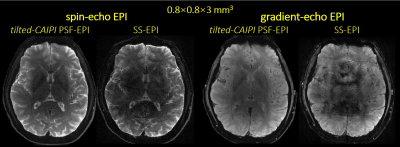
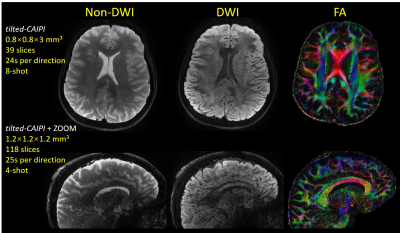
Fig.2 shows SE PSF-EPI data at RPSF= 6 & 22 from i) reduced-FOV, ii) conventional PSF-GRAPPA, and iii) PSF-GRAPPA with tilted-CAIPI, where only tilted-CAIPI can achieve good reconstruction at RPSF=22. Fig.3 demonstrates that single-shot-EPI (SS-EPI) at RPE=4 still contains strong distortions, while 8-shot PSF-EPI with tilted-CAIPI achieves distortion-free imaging with high SNR. Fig.4 shows T2- and T2*-weighted images from GE and SE acquisition where PSF-EPI shows markedly improved image quality when compared to SS-EPI. Fig.5 shows distortion-free and high-SNR diffusion-weighted images and FA maps from PSF-EPI with tilted-CAIPI.Discussion and Conclusion
The tilted-CAIPI method achieves dramatic acceleration for PSF-EPI through exploiting the inherent correlation in ky-kpsf with optimized under-sampling and B0-inhomogeneity-informed reconstruction. The ability of tilted-CAIPI to achieve fast distortion- and blurring-free imaging with high SNR was demonstrated, in which 1mm resolution range can be obtained in just 4-8 shots, 20s-30s for whole-brain T2/T2*-weighted imaging. More advanced reconstruction such as low-rank could be incorporated to further improve acceleration/reconstruction performance. A single calibration dataset can be used to reconstruction multiple imaging data as demonstrated in the GE, SE and DWI. The use of low-resolution GE scan at short TE can reduce whole-brain calibration scan to <20s.Acknowledgements
This work was supported in part by NIH research grants: R01EB020613, R01EB019437, R24MH106096, P41EB015896, and the shared instrumentation grants: S10RR023401, S10RR019307, S10RR019254, S10RR023043.References
1. Zeng H, Constable RT. Image distortion correction in EPI: comparison of field mapping with point spread function mapping. Magn. Reson. Med. 2002;48:137-146.
2. Robson MD, Gore JC, Constable RT. Measurement of the point spread function in MRI using constant time imaging. Magn. Reson. Med. 1997;38:733-740.
3. In MH, Posnansky O, Speck O. High-resolution distortion-free diffusion imaging using hybrid spin-warp and echo-planar PSF-encoding approach. NeuroImage 2017;148:20-30.
4. Zaitsev M, Hennig J, Speck O. Point spread function mapping with parallel imaging techniques and high acceleration factors: fast, robust, and flexible method for echo-planar imaging distortion correction. Magn. Reson. Med. 2004;52:1156-1166.
5. Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM. Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn. Reson. Med. 2006;55:549-556.
6. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn. Reson. Med. 2012;67:1210-1224.
7. Heidemann RM, Anwander A, Feiweier T, Knosche TR, Turner R. k-space and q-space: combining ultra-high spatial and angular resolution in diffusion imaging using ZOOPPA at 7 T. NeuroImage 2012;60:967-978.
8. Dong Z, Wang F, Reese TG, Manhard MK, Bilgic B, Wald LL, Guo H, Setsompop K. Fast Distortion-Free Diffusion Imaging using “tilted-CAIPI” PSF-EPI. Submitted to: In Proceedings of the 26th Annual Meeting of ISMRM 2018.
Figures