0210
Phase Encoded xSPEN: A High-Definition Approach to Volumetric MRI with Unusually High Acceleration Factors1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, Berkeley, CA, United States
Synopsis
xSPEN is a single-shot MRI approach whose timing and pre-acquisition hyperbolic phase, endow it with exceptional resilience to offsets. We recently introduced multi-scan, phase-encoded (PE) 3D xSPEN MRI which preserves this while increasing resolution along the PE (y) and slab-selection (z) dimensions. It is here shown that parallel receivers endow this 3D approach with unprecedented PE downsampling performances. This reflects xSPEN’s unusual kernel, whose hyperbolic phase couples the directly-sampled kz information with the y coil sensors. This mitigates the artifacts associated with a highly undersampled ky axis, as demonstrated by highly accelerated in vitro and human scans.
Introduction
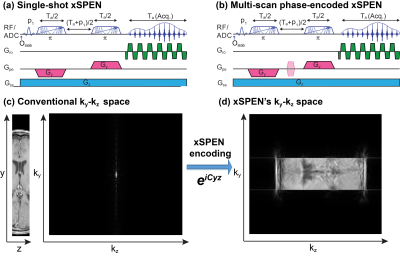
xSPEN (Fig. 1a) is a new approach to single-scan imaging endowed with unprecedented resilience to field distortions 1. xSPEN uses frequency-swept pulses under the action of bipolar ±Gy and constant Gz gradients, to impart a hyperbolic phase eiCyz modulation. This encoding kernel leads to a unique situation whereby Gz reads out y-axis profiles –and vice-versa. Phase-encoded xSPEN (Fig. 1b) is an extension of this single-shot protocol to volumetric 3D imaging 2, incorporating an additional ky loop. This simultaneously yields ky/kz data containing low and high frequency components, as well as transposed, low-resolution z/y images 2 (Figs. 1c, 1d). This study demonstrates that when multiple receivers are available, the method’s redundant information results in unique ky downsampling capabilities with minimal artifacts and excellent SNR. This allows one to complete 3D spin-echo-based brain scans with full Fourier multiplexing and sub-millimeter isotropic resolution in ≤2 min, using conventional, commercial scanners and coils.Methods
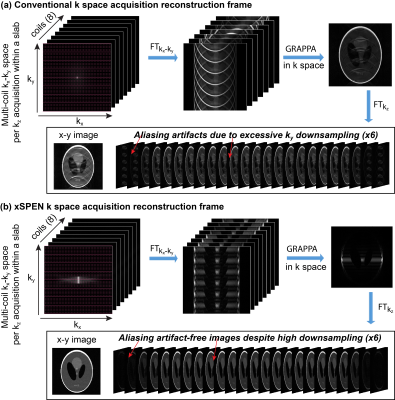
xSPEN’s eiCyz pre-encoding leads to a situation whereby the k-space sampling spreads from the localized 2D sinc of traditional MRI (Fig. 1c) into a 2D box-like shape (Fig. 1d). In traditional k-space acquisitions, the spatial dimensions are thus de-coupled, and the number of effective coil elements along a spatial dimension limits the downsampling that can be afforded along that axis: beyond this limit, SNR will rapidly drop and visible artifacts appear. By contrast, in xSPEN acquisitions where ky,kz are coupled by a bilinear phase, a particular kz value gives (low-resolution) insight about a specific position along the y profile. This kz-based y-information mitigates the aliasing artifacts that will appear if downsampling is applied in the ky dimension. Figure 2 demonstrates this by comparing the GRAPPA-based reconstruction3 of traditional k-space and of xSPEN acquisitions, using simulations that assume an acceleration factor of 6 along the PE dimension using a generic 8-channel head coil. In the traditional case, this ky downsampling leads to overlapping replicas of the full object, which are beyond the separation ability of multi-coil harmonics even after GRAPPA3. By contrast, the coupling arising in xSPEN means that for a specific kz, only local y contributions will be acquired by ky. Downsampling this variable will thus result, for a particular kz, in replicas of local y contributions and not of the full object. This filtering allows the GRAPPA reconstruction to yield artifact-free images, despite a highly downsampled (i.e., a highly accelerated) acquisition. (Notice that throughout this discussion xSPEN’s third, EPI-like ±kx sampling was ignored as it is conventionally sampled and decoupled form the other domains; it is intriguing to think what opportunities would arise if this weren’t the case).Results and Discussion
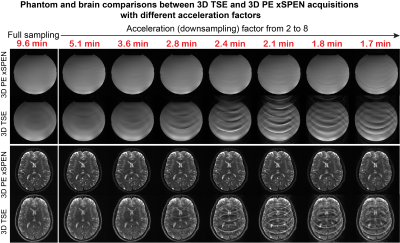
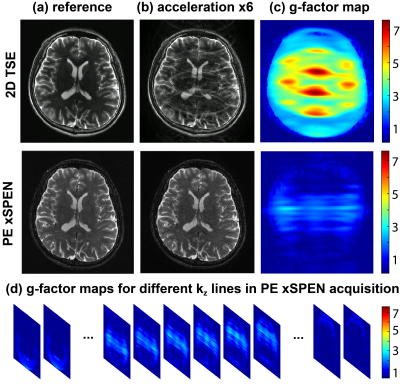
Experimental tests of these considerations were carried out on a 3T Siemens TrioTIM® using a 32-channels head coil on phantoms and on human volunteers; the latter following suitable written consent. Figure 3 compares the effects of downsampling in TSE4 vs PE xSPEN 3D acquisitions, using water and a human head as test cases. A slab thickness FOVz=12mm with 29 kz encodings was used, achieving an oversampling factor of 1.2 along this dimension. The echo train length in PE xSPEN was thus set 29, while the TSE turbo factor was also set to 29 in order to keep similar acquisition times for both sets of tests. The Figure clearly exemplifies what would happen if scan times were reduced in these experiments from 9.6 to 1.7 min, by increasing the ky downsampling. Notice the significant artifacts that appear in 3D TSE when downsampling exceeds ≈3. By contrast PE xSPEN remains nearly artifact-free till downsampling ca. 5x for the phantom, and brain scans remain artifact-free till reaching a downsampling factor of 7. Figure 4 exemplifies another aspect of PE xSPEN’s extreme downsampling ability, by comparing its g-maps5 with those of a 2D TSE acquisition for an acceleration of 6. Figure 4b clearly demonstrates the weaker artifacts appearing in the PE xSPEN (x,y) plane upon acceleration, while Fig. 4c rationalizes this by showing the much smaller g-factors of this new scheme. Figure 4d completes this picture by showing how these g-factor maps change for different kz acquisition points; although spatially- and kz-dependent, these g-factors always remain lower than those of TSE downsampled acquisitions.Conclusions
A new, highly accelerated approach to 3D MRI acquisitions based on phase encoded xSPEN was introduced, capable of delivering sub-millimeter resolutions in whole head 3D images within 2 min, with excellent SNR and free of aliasing artifacts. Further improvements including compressed sensing, SENSE-based reconstruction and multibanding, are currently in progress.Acknowledgements
Funding by the Israel Science Foundation grant 2508/17, ERC-2016-PoC grant # 751106, and the Kimmel Institute of Magnetic Resonance (Weizmann Institute) is acknowledged. ZZ thanks Israel’s Council of Higher Education and the Koshland Foundation for postdoctoral fellowships. ML acknowledges a Visiting Faculty Program Fellowship (Weizmann). We are also grateful to Dr. Sagit Shushan (Wolfson MC) and to the Weizmann MRI team (Edna Furman-Haran, Fanny Attar and Nachum Stern).References
1. Zhang Z., Seginer A ., Frydman L., Single-scan magnetic resonance imaging with exceptional resilience to field heterogeneities, Magn Reson Med 2017;77: 623–634
2. Zhang Z, Lustig M. Frydman L., High resolution imaging by phase encoded xSPEN MRI, Proceedings of 25th ISMRM, Honolulu, USA, April 22-27, 2017; Zhang Z, Lustig M. Frydman L., Phase-encoded xSPEN: A novel high-resolution volumetric alternative to RARE and spin-echo EPI, Magn Reson Med 2017 (in revision).
3. Griswold, M. A., P. M. Jakob, R. M. Heidemann, M. Nittka, V. Jellus, J. Wang, B. Kiefer and A. Haase. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2002;47: 1202-1210.
4. Yuan C, Schmiedl UP, Weinberger E, Krueck WR, Rand SD. Three-dimensional fast spin-echo imaging: Pulse sequence and in vivo image evaluation. J Magn Reson Imaging 1993;3:894-899.
5. Robson P.M., Grant A.K., Madhuranthakam A.J., Lattanzi R., Sodickson D.K., McKenzie C.A., Comprehensive quantification of signal‐to‐noise ratio and g‐factor for image‐based and k‐space‐based parallel imaging reconstructions. Magn Reson Med, 2008. 60: 895-907.
Figures