0208
Spin And Field Echo (SAFE) dynamic field correction in 3T fetal EPI1King's College London, London, United Kingdom, 2Physikalisch-Technische Bundesanstalt, Braunschweigh and Berlin, Germany
Synopsis
A method for echo planar imaging dynamic B0 field correction based on phase unwrapping is presented. For gradient-echo functional studies, the phase of the natively acquired images is used to estimate the accumulated B0-induced dephasing. For spin-echo diffusion, a matched echo planar imaging field echo navigator is acquired after the spin-echo readout so that motion-induced phase components can be subtracted before unwrapping. Application to both functional and diffusion in-vivo 3T fetal brain imaging is illustrated.
Introduction
In fetal brain imaging, breathing, motion and gas bubbles often provoke susceptibility-induced dynamic variations of the B0 field, which may disrupt the signal stability in the fetal brain boundaries (Fig. 1). This is especially the case at higher field strengths and for studies that rely on echo planar imaging (EPI), such as functional1 (fMRI) and diffusion2 (dMRI) imaging. In dMRI, magnitude-based registration approaches are of general use for dynamic distortion correction, with a few fetal examples3,4, but they may struggle near the signal to noise ratio (SNR) floor or in areas with limited contrast. In fMRI, phase unwrapping based methods have also been proposed5,6, but still with scarce fetal implementations7. This abstract contributes with an harmonized phase-based scheme for both dMRI and fMRI able to provide estimates of the field evolution at the spatio-temporal resolution of the native sequence.Methods
Test
data for fMRI and dMRI has been respectively acquired from 10 and 2
pregnant subjects (gestational ages 27-34 weeks) using a Philips 3T
Achieva scanner with a 32 channel cardiac coil. All subjects were
scanned supine, axially, and with anterior-posterior phase
encoding (PE). Simultaneous multi-slice (SMS) acceleration was
applied and complex data was reconstructed using a hybrid space
sensitivity encoding reconstruction8.
fMRI is acquired with a 2x SMS, 2x PE subsampling, ascending slice order, no half-scan, echo time TE=60ms,
voxel size Δ=2.2mm3,
and repeat time TR=2.8s. dMRI is acquired with 2x SMS, 2x
PE subsampling, odd-even slice interleave, and 0.75x half-scan. The
scanner software was modified to allow a dual hybrid echo comprised
of a standard spin-echo (SE) diffusion readout followed immediately
by a matched field echo (FE) readout. This spin and field echo (SAFE) method was
operated with TSE=70ms / TFE=135ms, Δ=2mm3,
four b-values at b={0,0.4,0.7,1}ms/μm2,
and TR=6.5s.
In this setting the SE
and FE are in the same distorted frame, and
complex phase subtraction is used to remove the component due
to motion during the diffusion gradient9,
so that phase-based dynamic B0 estimation techniques6
can be used in dMRI. Field estimation is based on a 3D+T
extension of the phase unwrapping max-flow/min-cut (PUMA) approach10. The unwrapped phase is low pass filtered to limit field singularities and the distortion is reversed by a conjugate phase reconstruction. When required in the experiments, per-shot motion correction11 is applied subsequently.
Results
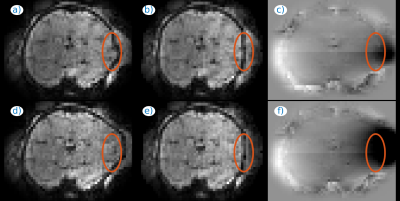
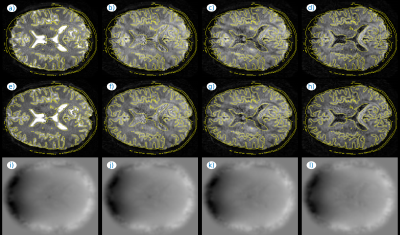
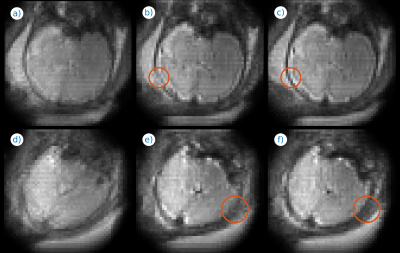
Fig. 1 illustrates the effect of distortion and motion correction at two different time instants of a fMRI series. The estimated B0 (Figs. 1c,f) changes substantially between the two time instants, which is consistent with the apparent changes in brain shape (Figs. 1a,d), largely corrected with our method (Figs. 1b,e). Fig. 2 includes an animation to illustrate the temporal stability of the signal after both corrections (bottom row) as compared with no corrections (top row). Fig. 3 shows the application of the method to adult brain dMRI acquired using the fetal protocol. A better match of the brain structures with the isolines from an anatomical scan is observed after (Figs. 3e-h) than before (Figs. 3a-d) correction, and the estimated field remains largely insensitive to the applied b-value (Figs. 3i-l). In Fig. 4 we show both magnitude (Figs. 4a-d) and phase (Figs. 4e-h) information from a dMRI fetal examination together with the estimated B0 (Figs. 4i-l) for different b-values. The estimations follow a similar trend, without the slice inconsistencies observed in the original phase data, although with an increased variance in low SNR regimes. In Fig 5, we provide examples in fetal dMRI acquired with opposite PE directions. Namely, we compare averaged unprocessed b=0 datasets (Figs. 5a,d), the result of only correcting for motion (Figs. 5b,e) and the combined result of motion and dynamic distortion correction (Figs. 5c,f). Areas enclosed in red show improved contrast (Fig. 5f versus 5e) and boundary localization (Fig. 5c versus 5b) when using the full model.
Discussion
The proposed phase-based method has shown promising results in correcting dynamic distortions in 3T fetal fMRI and dMRI scans, with the latter requiring dual SAFE data collection. In dMRI our method may be limited by the signal dropouts of FE versus SE data. However, for fetal application we take advantage of longer T2* in the fetal brain12, and so far have not encountered unrecoverable signal dropouts at our ΔTE=65ms. Future work will focus on testing different phase unwrapping approaches for more versatile and robust application of our approach.Conclusions
A phase-based technique for dynamic distortion correction in fMRI has been extended to dMRI corrections using the SAFE protocol. Preliminary results indicate potential in demanding distortion correction regimes such as 3T fetal brain imaging.Acknowledgements
This work received funding from the European Research Council under the European Union’s Seventh Framework Programme (FP7 2007-2013) ERC grant agreement no. [319456] (dHCP project), and was supported by the Wellcome EPSRC Centre for Medical Engineering at Kings College London [WT 203148/Z/16/Z], MRC strategic grant [MR/K006355/1] and by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.References
1. Ferrazzi, G, Murgasova, MK, Arichi, T, et al. Resting state fMRI in the moving fetus: A robust framework for motion, bias field and spin history correction. NeuroImage. 2014;101:555-568.
2. Oubel, E, Koob, M, Studholme, C, et al. Reconstruction of scattered data in fetal diffusion MRI. Med Image Anal. 2012; 16:28-37.
3. Kuklisova-Murgasova, M, Estrin GL, Nunes, RG, et al. Distortion correction in fetal EPI using non-rigid registration with a Laplacian constraint. IEEE Trans Med Imag. 2017, in press.
4. Hutter, J, Christiaens, D, Deprez, M, et al. Dynamic Field Mapping and Motion Correction Using Interleaved Double Spin-Echo Diffusion MRI. MICCAI Part I, LNCS 10433, 2017; 523-531.
5. Hutton, C, Bork, A, Josephs, O, et al. Image Distortion Correction in fMRI: A Quantitative Evaluation. NeuroImage. 2002; 16(1):217-240.
6. Dymerska, B, Poser, BA, Barth, M. A method for the dynamic correction of B0-related distortions in single-echo EPI at 7T , NeuroImage. 2017, in press.
7. Sehamani, S, Blazejewska, AI, McKown, S, et al. Detecting Default Mode Networks in Utero by Integrated 4D fMRI Reconstruction and Analysis. Human Brain Mapping. 2016; 37:4158-4178.
8. Zhu, K, Dougherty, RF, Wu H, et al. Hybrid-Space SENSE Reconstruction for Simultaneous Multi-Slice MRI. IEEE Trans Med Imag. 2017; 35(8):1824-1836.
9. Anderson, A, Gore, JC. Analysis and correction of motion artifacts in diffusion weighted imaging, Magn Reson Med. 1994, 32:379–387.
10. Bioucas-Dias, JM, Valadão, G. Phase Unwrapping via Graph Cuts. IEEE Trans Image Proc. 2007; 16(3): 698-709.
11. Cordero-Grande, L, Hughes, EJ, Hutter, J, et al. Three-Dimensional Motion Corrected Sensitivity Encoding Reconstruction for Multi-Shot Multi-Slice MRI: Application to Neonatal Brain Imaging . Magn Reson Med. 2017, in press.
12. Blazejewska, AI, Seshamani, S, McKown, SK, et al. 3D In Utero Quantification of T2* Relaxation Times in Human Fetal Brain Tissues for Age Optimized Structural and Functional MRI. Magn Reson Med. 2017; 78:909-916.
Figures