0178
Multi-delay arterial spin labeling (ASL) more accurately detects hypoperfusion in Moyamoya disease: comparison with a normative PET/MRI database1Radiology, Stanford University, Stanford, CA, United States, 2GE Healthcare, Menlo Park, CA, United States, 3Neurosurgery, Stanford University, Stanford, CA, United States
Synopsis
We directly compared multi-delay arterial spin labeling (ASL) and standard ASL measurements of cerebral blood flow (CBF) to simultaneously acquired [15O]-PET scans on hybrid PET/MRI in Moyamoya disease. For these Moyamoya patients (N=15) with extremely long arterial transit times, multi-delay ASL outperforms standard ASL in regional correlation and reduces bias relative to PET. We also constructed a voxelwise, normative CBF database based on healthy controls (N=15) with PET/MRI, and identified regions of hypoperfusion in frontal and parietal regions of patients. Multi-delay ASL is more specific to areas of Moyamoya hypoperfusion (more similar to PET), whereas standard ASL overestimates these areas due to low signal.
Introduction
Despite recent efforts to standardize arterial spin labeling (ASL) MRI for perfusion imaging1, typical ASL protocols often fail in patients with long arterial transit times (ATTs)2. In Moyamoya disease, patients benefit from cerebral blood flow (CBF) assessment to decide on surgical revascularization, but are prone to ASL signal loss that mimics low perfusion due to extremely long ATT3. Furthermore, ASL lacks validation with a gold standard (PET), since natural fluctuations in brain physiology render comparisons between different modalities challenging4. This study uses simultaneous PET/MRI to compare multi-delay and standard ASL to [15O]-water PET in Moyamoya patients; and investigates whether ASL and PET identify similar areas of pathophysiology relative to a normative database from healthy controls.Methods
Simultaneous time-of-flight 3T PET/MRI (GE Healthcare Signa) was acquired in 15 healthy volunteers (ages 27-62 years, 8 female) and 15 patients with Moyamoya disease (ages 25-52 years, 11 female). Four patients had unilateral disease and the rest had bilateral stenosis or occlusion. PET imaging of CBF with injection of [15O]-water (550-925 MBq) was performed simultaneously with standard ASL (single post-label delay of 2025ms) and sequential multi-delay ASL (5 delays, 700-3000ms). ASL scan parameters included pseudo-continuous labeling with label duration of 1450ms in standard-delay and 2000ms in multi-delay; echo time=10.7ms; bandwidth=62.5kHz; and 3D stack-of-spiral readout. A T1-weighted anatomical scan was acquired for registration and dynamic susceptibility contrast was performed at the end to assess time-to-maximum (Tmax) delays.
Dynamic [15O]-water PET frames were reconstructed (30x1s, 10x3s, 12x5s, 12x10s) and an image-derived input function was created for each individual from the cervical arteries, correcting for spill-over effects with the true arterial volume (segmented on MR angiogram)5. Quantitative PET CBF (ml/100g/min) maps then were generated by fitting a one-tissue kinetic model with weighted linear regression in PMOD software. ASL CBF maps were quantified with a one-compartment model for standard ASL; and a two-compartment model for multi-delay ASL (T1,tissue=1200ms) including correction for estimated ATT6. Perfusion images were registered to the Montreal Neurological Institute template with Advanced Normalization Tools (ANTs) software, and regional CBF values were estimated in 10 perfusion territories per hemisphere based on ASPECTS7.
Results
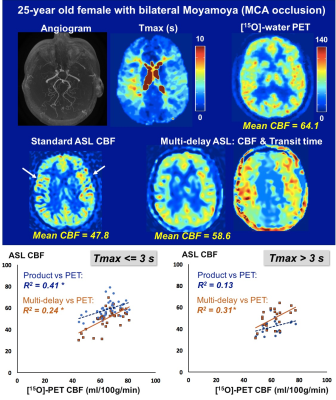
Figure 1 shows representative PET/MRI perfusion images in a patient with bilateral Moyamoya disease. Standard ASL showed areas of reduced CBF relative to PET due to long ATTs characteristic of Moyamoya pathology, as well as focal hotspots indicative of slow flow in large vasculature. Multi-delay ASL visually improved the CBF uniformity along the cortex and showed stronger correlation with PET in areas of longer delays (Tmax>3s).
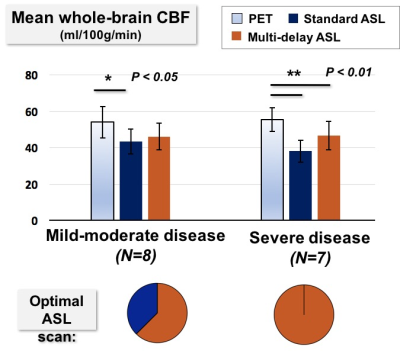
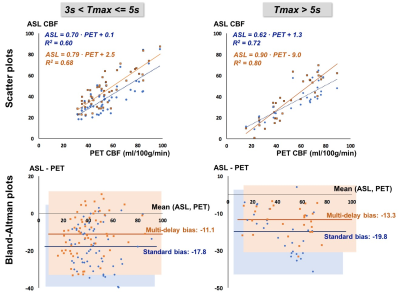
Patients were classified into mild-moderate disease (N=8) or severe disease (N=7) based on Tmax delays. While standard ASL underestimated CBF in both groups, whole-brain CBF from multi-delay ASL did not differ from PET values in mild-moderate cases, suggesting adequate correction for ATT (Figure 2). In all severe cases, multi-delay ASL outperformed standard ASL as the “optimal ASL scan” for regional correlation with PET. Figure 3 aggregates across all patients the cortical regions with 3s< Tmax <=5s and with Tmax>5s. Multi-delay ASL improved correlation with PET (R2=0.80, p<10-5) and reduced CBF bias on Bland-Altman plots, especially in regions with Tmax>5s.
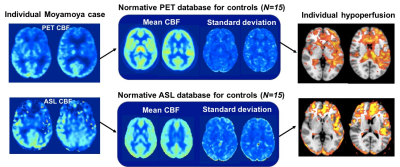
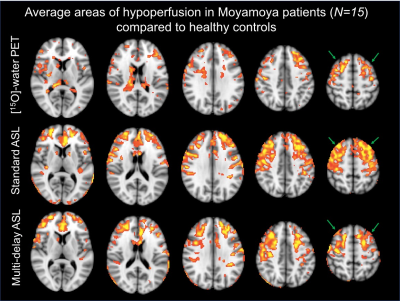
Normative CBF databases including mean CBF and standard deviation (SD) per voxel were generated from the healthy control images separately for PET and for each ASL sequence (Figure 4). In each Moyamoya case, after registration to template space, the individual’s PET scan was compared to the PET database and each ASL scan was compared to the corresponding ASL database. This enabled voxelwise identification of hypoperfusion in the patient, defined as 2 SDs below the mean CBF in healthy controls. Figure 5 shows average areas of hypoperfusion across all patients (N=15) relative to healthy controls, localized bilaterally in the anterior and middle cerebral artery territories. Standard ASL overestimates the regions affected by Moyamoya disease compared to PET, whereas multi-delay is more specific to true hypoperfusion (Figure 5).
Discussion
CBF quantification with ASL and comparison with a normative database identified regions of hypoperfusion in frontal and parietal regions that are consistent with Moyamoya pathology8, mostly bilateral disease in our cohort. Not surprisingly, standard ASL overestimated the areas of hypoperfusion compared to PET, reflecting that low ASL signal in standard protocols may be incorrectly interpreted as low CBF. We showed that multi-delay ASL acquisition improved the detection specificity for hypoperfusion in Moyamoya disease, and that its improved CBF correlation with PET is especially critical for longer Tmax delays > 5s. Despite this improvement, multi-delay ASL still underestimated absolute CBF levels in severe cases and may require an extended post-label delay range (up to 5s)9 or alternative velocity-selective strategies10 to accurately quantify CBF in challenging cerebrovascular cases.Acknowledgements
GE Healthcare, Stanford Neuroscience Institute Interdisciplinary Scholar AwardReferences
- Alsop D.C., Detre J.A., Golay X., et al. Recommended Implementation of Arterial Spin Labeled Perfusion MRI for Clinical Applications: A consensus of the ISMRM Perfusion Study Group and the European Consortium for ASL in Dementia. Magnetic resonance in medicine. 2015;73(1):102-116. doi:10.1002/mrm.25197.
- Noguchi, T., Kawashima, M., Irie, H., Ootsuka, T., Nishihara, M., Matsushima, T., & Kudo, S. (2011). Arterial spin-labeling MR imaging in moyamoya disease compared with SPECT imaging. European journal of radiology, 80(3), e557-e562.
- Goetti, R., Warnock, G., Kuhn, F. P., Guggenberger, R., O'Gorman, R., Buck, A., ... & Scheer, I. (2014). Quantitative cerebral perfusion imaging in children and young adults with moyamoya disease: comparison of arterial spin-labeling–MRI and H2 [15O]-PET. American Journal of Neuroradiology, 35(5), 1022-1028.
- Fan, A. P., Jahanian, H., Holdsworth, S. J., & Zaharchuk, G. (2016). Comparison of cerebral blood flow measurement with [15O]-water positron emission tomography and arterial spin labeling magnetic resonance imaging: a systematic review. Journal of Cerebral Blood Flow & Metabolism, 36(5), 842-861.
- Khalighi, M. M., Deller, T. W., Fan, A. P., Gulaka, P. K., Shen, B., Singh, P., Park J.H., Chin F.T. & Zaharchuk, G. (2017). Image-derived input function estimation on a TOF-enabled PET/MR for cerebral blood flow mapping. Journal of Cerebral Blood Flow & Metabolism, 0271678X17691784.
- Dai W, Robson PM, Shankaranarayanan A, Alsop DC. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn Reson Med. 2012;67:1252–1265. doi: 10.1002/mrm.23103.
- Kim JJ, Fischbein NJ, Lu Y, Pham D, Dillon WP. Regional angiographic grading system for collateral flow: correlation with cerebral infarction in patients with middle cerebral artery occlusion. Stroke. 2004;35:1340–1344. doi: 10.1161/01.STR.0000126043.83777.3a.
- Nariai, T., Matsushima, Y., Imae, S., Tanaka, Y., Ishii, K., Senda, M., & Ohno, K. (2005). Severe haemodynamic stress in selected subtypes of patients with moyamoya disease: a positron emission tomography study. Journal of Neurology, Neurosurgery & Psychiatry, 76(5), 663-666.
- Qiu, D., Straka, M., Zun, Z., Bammer, R., Moseley, M. E., & Zaharchuk, G. (2012). CBF measurements using multidelay pseudocontinuous and velocity‐selective arterial spin labeling in patients with long arterial transit delays: Comparison with xenon CT CBF. Journal of Magnetic Resonance Imaging, 36(1), 110-119.
- Fan, A. P., Guo, J., Khalighi, M. M., Gulaka, P. K., Shen, B., Park, J.H. Gandhi H, Holley D. Rutledge O., Singh P Haywood, T., Steinberg G.K., Chin F.T., Zaharchuk G. (2017). Long-Delay Arterial Spin Labeling Provides More Accurate Cerebral Blood Flow Measurements in Moyamoya Patients: A Simultaneous Positron Emission Tomography/MRI Study. Stroke, 48(9), 2441-2449.
Figures