0170
Spatially-selective excitation using a tailored nonlinear ΔB0 pattern generated by an integrated multi-coil ΔB0/Rx array1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Faculty of Psychology and Neuroscience, Maastricht University, Maastricht, Netherlands, 5Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Synopsis
Multi-coil ΔB0 shim arrays have recently been used for zoomed imaging of target ROIs in mice, providing improved acquisition efficiency without the high SAR, long RF pulses, and other limitations of conventional selective excitation methods that rely solely on linear gradients. We extend this work to zoomed 3D EPI in humans using an integrated ΔB0/Rx array coil to dynamically switch-on a static, spatially-tailored nonlinear ΔB0 pattern during RF excitation. Proof-of-concept selective excitations of the occipital visual cortex and the peripheral cerebrum are shown, with strong correspondence to the target patterns. Four-fold in-plane undersampled EPI of occipital visual cortex demonstrates the method’s potential for efficient high-resolution neuroimaging.
Introduction
High-resolution functional and anatomical imaging provides improved depiction of small brain structures. However, full encoding results in prolonged acquisition times, introducing distortion and blurring artifacts in functional imaging and motion vulnerability in anatomical imaging1. Methods such as Inner Volume Imaging and Outer Volume Suppression have been proposed to overcome this limitation by only exciting the target ROI2-6. But these methods typically require longer RF pulse durations and/or higher SAR. Pulse durations can be reduced using parallel transmit excitation7-8, but this involves complex pulse optimization using subject-specific B1+ field distributions that include SAR and B0-robustness constraints.
An entirely different approach is to use arrays of independently-driven multi-coil (MC) ΔB0 shim loops, as recently demonstrated for selective excitation in mouse brain9. The MC ΔB0 array generates a nonlinear ΔB0 pattern in the ROI during RF excitation, such that a given bandwidth of the RF pulse is on-resonance for regions inside the target ROI, but not outside.
Here, we extend this approach to zoomed 3D EPI acquisitions in humans using an integrated ΔB0/Rx array coil originally developed for high spatial-order B0 shimming, in which the B0 shim and receive functions use the same set of wire loops on a close-fitting helmet10. The array is well-suited for selective excitation because (a) it has many degrees of freedom for field control, (b) its coil currents can be switched quickly (i.e., energized differently during the excitation and readout phase), and (c) it provides high receive sensitivity and parallel imaging capability required for high-resolution imaging. For proof-of-concept, we excite two target ROIs in a 3D EPI acquisition: occipital visual cortex and the outer peripheral cerebrum. The choice of a peripheral cerebrum ROI is motivated by recent findings that sparse images can be highly-undersampled with reduced g-factor penalties11.
Methods
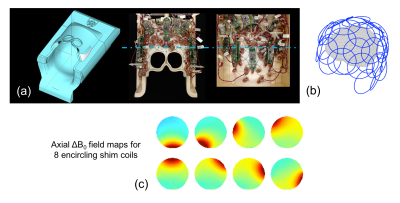
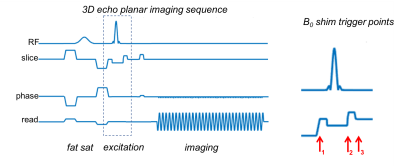
Two volunteers were scanned on a Siemens Skyra 3T scanner using both conventional slab excitation and MC-enabled selective excitation followed by 3D multi-shot EPI (sixty 1.5mm partitions). Figure 1 shows the integrated ΔB0/Rx coil, with 32 RF receive channels and 31 ΔB0 shim channels sharing the same set of loops. Second-order scanner shims were applied and baseline ΔB0 field maps were acquired. A min-max algorithm was used to compute MC ΔB0 patterns based on an objective function enforcing frequency separation between the target and excluded regions, subject to constraints on the maximum currents (3.5A/coil or 40A/total). The RF pulse bandwidth and center frequency were set to overlap with the B0 pattern in the target ROI. The shim hardware was controlled from the 3D EPI pulse sequence (Figure 2) using 10μs TTL pulses applied 500μs before each windowed-sinc RF excitation pulse, at the end of the RF pulse (during which the negative current was applied to “re-wind” the spin phase, and one half the pulse duration after the end (to end the re-wind); the MC shim coils were turned off during the EPI readout.Results
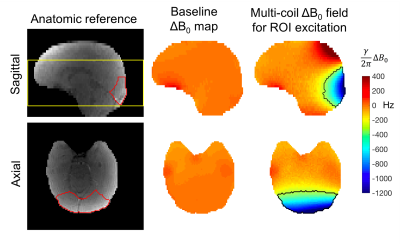
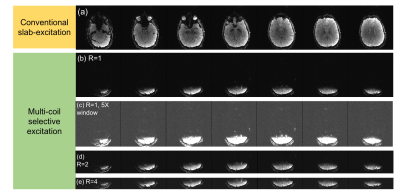
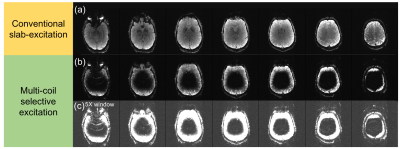
Figure 3 shows the occipital visual cortex anatomic mask overlaid on a reference image and the ΔB0 calculated by the optimizer to match this ROI. While the agreement is not perfect, the optimizer achieves spectral separation of the target and excluded regions to prevent unwanted signal from aliasing into the target ROI. Figure 4 shows the corresponding raw images over a 9cm slab acquired for both slab selection and MC-enabled zoom with R={1,2,4} undersampling along the primary (in-plane) phase encode direction. Even without performing parallel image reconstruction, very little aliasing is observed, even in images with a 5-fold narrower display window. Figure 5 shows selective excitation of the peripheral cerebrum. The excited region follows the contours of the cerebral surface with high fidelity over most of the slab, with some artifacts in the most superior and inferior partitions. These artifacts may be addressed using more sophisticated phase correction navigators12.Discussion
In the peripheral cerebrum acquisition, the most inferior and superior partitions show some artifacts and deviations from the target shape, illustrating the limitations of this particular 31ch ΔB0 shim coil geometry. Performance is especially degraded in the deep brain/brainstem where the coil loops do not fully encircle the head (see Fig. 1), a limitation that could be overcome in future coil designs. In future work, standard windowed-sinc RF pulses will be replaced by pulses with sharper transition bands, providing clearer demarcation of the excited ROI.
We show MC-enabled zoomed 3D EPI using an integrated ΔB0/Rx array that provides both ΔB0 field control for selective excitation as well as high RF sensitivity for high-resolution imaging. We anticipate that the technique will be especially useful at ultra-high field (7T), where the improved SNR and contrast-to-noise ratio enable very high-resolution zoomed imaging of targeted structures.
Acknowledgements
We thank Ned Ohringer for assistance acquiring data on human volunteers. This work was supported in part by the NIH NIBIB (grants K99-EB021349, P41-EB015896, R01-EB019437), by the BRAIN Initiative (NIH NIMH grant R01-MH111419), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-OD010364, S10-RR023401, S10-RR023043, S10-RR019371.References
- Setsompop K, Feinberg DA, Polimeni JR. Rapid brain MRI acquisition techniques at ultra-high fields. NMR in Biomed., 2016;29:1198-1221.
- Feinberg A, Hoenninger JC, Crooks LE, Kaufman L, Watts JC, Arakawa M. Inner volume MR imaging: technical concepts and their application. Radiology 1985;156:743-747.
- Luo Y, de Graaf RA, Delabarre L, Tannus A, Garwood M. BISTRO: an outer-volume suppression method that tolerates RF field inhomogeneity. Magn. Reson. Med. 2001; 45:1095-1102.
- Heidemann RM, Ivanov D, Trampel R, Fasano F, Pfeuffer J, Turner R. Zoomed GRAPPA (ZOOPPA) for functional MRI. Int. Soc. Magn. Res. Med. 2010, p. 2889.
- Finsterbusch J. Simultaneous functional MRI acquisition of distributed brain regions with high temporal resolution using a 2D selective radiofrequency excitation. Magn. Reson. Med. 2015;73:683-691.
- Mooiweer R, Sbrizzi A, Raaijmakers AJE, van den Berg CAT, Luijten PR, Hoogduin H. Combining a reduced field of excitation with SENSE-based parallel imaging for maximum imaging efficiency. Magn. Reson. Med. 2017;78:88-96.
- Davids M, Schad LR, Wald LL, Guerin B. Fast three-dimensional inner volume excitations using parallel transmission and optimized k-space trajectories. Magn. Reson. Med. 2016;76(4):1170-1182.
- Poser BA, Kaas AL, Wiggins CJ, Uludag K, Desmond HYT. Dual region-selective spiral pTx excitation for digit mapping fMRI in motor cortex and cerebellum. ISMRM 2017, p. 588.
- Rudrapatna SU, de Graaf R, Nixon T, Juchem C. Multi-dimensional reduced field-of-view excitation by integrated RF pulse and DYNAMITE B0 field design. Int. Soc. Magn. Res. Med. 2016, p. 1010.
- Stockmann JP, Witzel T, Keil B, Polimeni JR, Mareyam A, LaPierre C, Setsompop K, Wald LL. A 32-channel combined RF and B0 shim array for 3T brain imaging. Magn. Reson. Med. 2016;75(1):441-51
- Gallichan D, Marques JP, Gruetter R. Retrospective correction of involuntary microscopic head movement using highly accelerated fat image navigators (3D FatNavs) at 7T. Mad. Reson. Med. 2016;75(3):1030-39.
- Hoge WS, Polimeni JR. Dual-polarity GRAPPA for simultaneous reconstruction and ghost correction of echo planar imaging data. Magn. Reson. Med. 2016;76(1):32-44.
Figures