0164
Neural-network discrimination of cardiac disease from 31P MRS measures of myocardial creatine kinase energy metabolism1Radiology, Division of MR Research, Johns Hopkins University, Baltimore, MD, United States, 2Medicine, Division of Cardiology, Johns Hopkins University, Baltimore, MD, United States, 3Radiology, Division of MR Resaerch, Johns Hopkins University, Baltimore, MD, United States
Synopsis
Myocardial energy demands are the highest in the body and cardiac metabolism is altered in common diseases. Only phosphorus magnetic resonance spectroscopy (MRS) can measure ATP and creatine-kinase (CK) metabolism, a primary reserve of ATP, noninvasively in the human heart. Here, neural-network analysis is used to test whether the combination of 31P MRS measurements of phosphocreatine and [ATP] concentrations, the CK reaction-rate and its ATP flux, can discriminate cardiac diseases among prior study data from 178 subjects. We find that a three-layer neural-network adequately discriminates diseases without over-training, suggesting that heretofore unidentified differences in CK metabolism may underlie cardiac disease.
Purpose
Adenosine triphosphate (ATP) is critical for cardiac viability and function. Cardiac metabolism is often altered in common diseases, but is not used clinically to distinguish conditions like heart failure (HF), dilated cardiomyopathy (DCM), hypertrophic disease (HD) including left ventricular (LV) hypertrophy (LVH), or myocardial infarction (MI). The heart’s primary chemical energy reserve is the creatine kinase (CK) reaction, which generates ATP from adenosine diphosphate by cleaving phosphocreatine (PCr), with a pseudo-first-order forward reaction-rate kf s-1, and flux-rate for generating ATP, FATP= kf [PCr] mmol/kg/s.
Many phosphorus (31P) cardiac MRS patient studies have reported altered CK metabolism in heart disease(1), and two studies suggest that 31P MRS measures of compromised CK energy may independently predict subsequent cardiac events(2,3). The present study uses a neural-network to test whether multi-parametric 31P MRS measurements of [PCr] and [ATP] concentrations, kf and FATP from prior patient studies can be used to differentiate healthy controls (C) and cardiac disease (HF, AMI, HD, DCM).
Methods
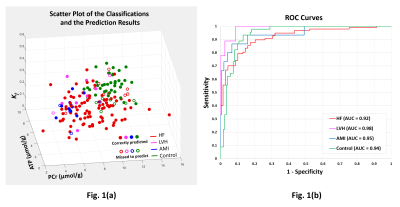
Anterior LV [PCr], [ATP], kf and FATP data were acquired from 178 institutionally-approved 31P MRS studies performed between 2001-2013 on General Electric 1.5T or Philips 3T MRI/MRS systems on C (n=45), nonischemic HF (n=109, including DCM, and HD), and LVH (n=10) and anterior MI (AMI, n=15) patients without HF(3-8). Diagnoses and New York Heart Association (NYHA) HF class were assessed at the time of MRS. Concentrations were measured using internal water or external phosphate referencing (9,10) and kf using ‘FAST’(11) or ‘TRiST’(12) methods. Fig. 1(a) plots the data as a function of the 3 independent variables [PCr], [ATP] and kf.
The neural-network, developed in MATLAB 2017a (MathWorks, Inc), was limited to a stack of two auto-encoder layers and a ‘softmax’ layer to avoid over-fitting. All of the data were used to train the network using the ‘leave-one-out’ cross-validation method(13) to fine-tune the network for uniform performance across the training set, with low L2-regularization to avoid over-training. Cross-validation results were captured as confusion matrices and receiver operating characteristic (ROC) curves.
Three classification tests were performed. In the first, the network was trained to recognize data from HF patients (including those with DCM and HD), LVH and AMI patients without HF, and Controls. Because LVH was associated with both HF and non-HF patients, 12 patients with both LVH and HF were removed from the training to improve prediction of non-HF LVH.
For the second classification test, a subset of those HF (n=60) patients diagnosed with DCM or with HD were used to train a network of the same layers but with the number of output classes reduced to match the two output classifications. The third test was performed using all of the HF data to test whether the neural-network could predict NYHA HF class based on the 31P MRS measurements alone.
Results
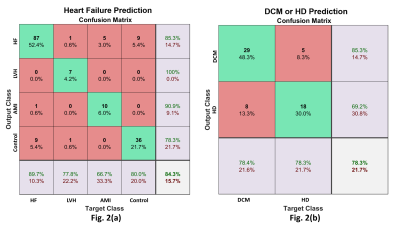
The classification results are color-coded in the scatter plot (Fig 1a) and show that the network can resolve data clusters (HF, LVH without HF, MI without HF, and C) in 3-dimensions, except where data points from one class are surrounded by data from other classes. This is a characteristic of networks that are not over-trained (to recognize all data points). The ROC curves are in Fig 1(b). The area-under-the-curve (AUC) for each class is ≥0.92. Fig 2(a) shows the corresponding confusion-matrix whose overall true positive success rate was 84%.
Fig 2(b) presents the confusion matrix for the second test on differentiating DCM from HD among HF patients. The overall true positive detection rate is 78%. The false positive rate is 22%. The results from the HF NYHA classification test showed that the network could successfully predict NYHA class 1 (87.5%), but the success rates for class 2 and higher were poorer, with data often misclassified as class I.
Discussion
This study demonstrates that a neural-network can successfully resolve cardiac disease, specifically: HF, from non-HF LVH, from non-HF MI, from healthy heart; based solely on multi-parametric 31P MRS measurements of CK metabolism. Moreover, the measures of [ATP], [PCr], kf and FATP used, relate directly to the heart’s energy reserve, vital to cardiac function. That these parameters can be used to resolve different disease types, suggests subtle differences specific to the underlying CK metabolism, that have heretofore not been specifically identified but which the neural network can detect.
Although data are presently limited in some disease sub-categories, a virtue of the neural-network approach is its ability to adapt and improve accuracy as more data are added. Data-augmentation approaches can also be used to complement real data that are harder to obtain.
Acknowledgements
Supported by AHA grant 13GRNT17050100 and NIH grant R01 HL61912.References
1. Bottomley PA. MRS Studies of Creatine Kinase Metabolism in Human Heart. eMagRes, 2016, Vol 5: 1183–1202. DOI 10.1002/9780470034590.emrstm1488.
2. S. Neubauer, M. Horn, M. Cramer, K. Harre, J. B. Newell, W. Peters, T. Pabst, G. Ertl, D. Hahn, J. S. Ingwall, K. Kochsiek, Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation 1997; 96:2190–2196.
3. Bottomley PA, Panjrath GS, Lai S, Hirsch GA, Wu K, Najjar SS, Steinberg A, Gerstenblith G, Weiss RG. Metabolic Rates of ATP Transfer Through Creatine Kinase (CK Flux) Predict Clinical Heart Failure Events and Death. Sci Transl Med. 2013;5:215re3-215re3. doi/10.1126/scitranslmed.3007328.
4. Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102:808-13.
5. Hirsch GA, Bottomley PA, Gerstenblith G, Weiss RG. Allopurinol Acutely Increases Adenosine Triphospate Energy Delivery in Failing Human Hearts. J Am Coll Cardiol 2012:802–8.
6. Smith CS, Bottomley PA, Schulman SP, Gerstenblith G, Weiss RG. Altered Creatine Kinase adenosine triphosphate kinetics in failing hypertrophied human myocardium. Circulation 2006; 114:1151-1158.
7. Bottomley PA, Wu KC, Gerstenblith G, Schulman SP, Steinberg A, Weiss RG. Reduced myocardial creatine kinase flux in human myocardial infarction: An in vivo phosphorus magnetic resonance spectroscopy study. Circulation 2009; 119: 1918-24.
8. Abraham MR, Bottomley PA, Dimaano VL, Pinheiro A, Steinberg A, Traill TA, Abraham TP, Weiss RG. Creatine kinase adenosine triphosphate and phosphocreatine energy supply in a single kindred of patients with hypertrophic cardiomyopathy. Am J Cardiol 2013; 112: 861-866.
9. Bottomley PA, Atalar E, Weiss RG. Human cardiac high-energy phosphate metabolite concentrations by 1D-resolved NMR spectroscopy. Magn Reson Med 1996; 35: 664-670.
10. El-Sharkawy A-MM, Gabr RE, Schär M, Weiss RG, Bottomley PA. Quantification of human high-energy phosphate metabolite concentrations at 3 T with partial volume and sensitivity corrections. NMR Biomed 2013;26:1363–71.
11. Bottomley PA, Ouwerkerk R, Lee RF, Weiss RG. Four angle saturation transfer (FAST) method for measuring creatine kinase reaction rates in vivo. Magn Reson Med 2002; 47: 850-863.
12. Schär M, El-Sharkawy A-MM, Weiss RG, Bottomley PA. Triple repetition time saturation transfer (TRiST) 31P spectroscopy for measuring human creatine kinase reaction kinetics. Magn Reson Med 2010;63:1493–501.
13. Li K-C. Asymptotic optimality for Cp, CL, cross-validation and generalized cross-validation: discrete index set. Ann Stat 1987;15:958–75.
Figures