0153
Probing temporal information in fast-TR fMRI data during attention modulations1CMRR, University of Minnesota, Minneapolis, MN, United States
Synopsis
The introduction of fast-TRs has allowed for explorations of temporal features in fMRI data. Further, the ability to concurrently retain relatively high degrees of spatial precision as well as large volume coverage, while also maintaining high SNR efficiency, could provide unprecedented axis to the human brain. In this work we explore the possibility of exploiting the temporal specificity of fMRI using a temporal multi-voxel pattern analysis and high temporal resolution 7T fMRI data during attention modulations.
Introduction
The popularity of fMRI is traditionally related to the spatial precision and large volume coverage with which functional images can be recorded non-invasively. However, due to the sluggishness (several seconds) of the Blood Oxygen Level Dependent (BOLD, 1), exploring temporal features has not been popular. With the advent of Ultra High Field (UHF) fMRI (i.e. 7 Tesla and above) and the development of more SNR efficient parallel accelerations, it is now possible to record BOLD signals across the brain with unprecedented spatial-temporal resolutions (<1 second). It remains to be seen, though, whether the gains from these ultra-fast measurements will be primarily from statistical power, or whether they can be exploited for unraveling fast temporal dynamics of neural processing. Recently a number of studies have reported that the BOLD signal carries neural information on a time-scale faster than previously conceived (e.g. up to 1 Hz., 2); and that using fast repetition times (TR) (e.g. ~500 ms) it is possible to extract more precise information about stimulus dimensions coded by a specific cortical region (3). Further, the possibility of measuring such fast processes with fMRI, while also maintaining a relatively high spatial resolution over a large volume, could shed light on a number of neuroscience questions that remain largely unresolved, such as inter- and intra-area communication related to feed-forward and feed-back signals. Attention is known to modulate early neuro-temporal dynamics (e.g. ~50-100 ms after stimulus onset, 4) and represents an ideal candidate against which to test the temporal precision of the BOLD signal. Here, we recorded GE BOLD fMRI at 7T from human participants following the presentation of visual stimuli, while manipulating attentional demands.Methods
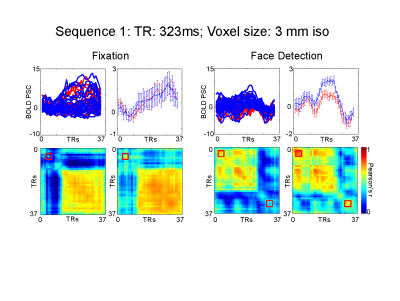
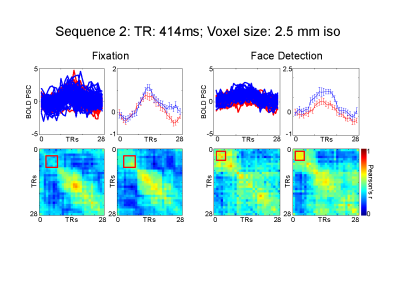
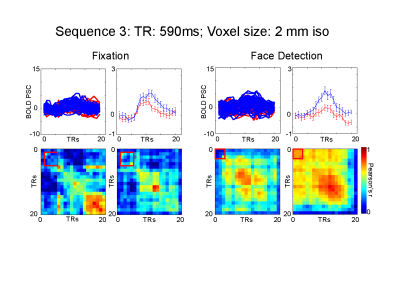
We used face stimuli subtending approximately 9 degrees of visual angle. We modulated the phase coherence of the images to create 9 visual conditions, ranging from 0% phase coherence (i.e. pink noise) to 45% phase coherence in incremental steps of 5%. Stimuli were presented for 2 seconds followed by a 2 seconds fixation period, with 10 % black trials. A fixation cross (subtending approximately 1 degree of visual angle), changing color every 250 ms was held constant in the middle of the screen. Participants performed either a face detection or fixation task, requiring them to respond to a specific color change. The face detection task directed attention towards the face stimuli, while the fixation task away from the faces. Tasks were blocked by runs and stimuli were identical across tasks. We tested 3 fMRI protocols using a 32 ch. Coil on a Siemens 7T : 1) 3 mm iso voxels; TR: 323 ms; FA: 31; 45 Slices; 2) 2.5 mm iso voxels; TR: 414 ms; FA: 35; 55 Slices; 3) 2 mm iso voxels; TR:590 ms; FA: 41; 70 Slices. In-plane and slice acceleration factors were kept constant: MB=5, IPAT= 2. Analysis: We localized rFFA using a standard face localizer. All analyses were confined within this ROI. We sorted the conditions according to participants’ behavioral response during the face detection task. Condition 1 included phase coherence levels for which a face was perceived for at least 70% of the trials; and condition 2, where no face was perceived for at least 70% of the trials. We implemented standard univariate FIR analysis and single trial temporal MVPA (tMVPA – measuring the synchrony of multi-voxel patterns across all time points - 5) to test latency and amplitude differences across conditions and tasks.Results
Univariate analysis: For all sequences, we observed significantly (p<.05 FDR corrected) larger amplitude for the face compared to the no-face condition at the peak of the HRF (~ 6 seconds after stimulus onset) during both tasks, with the sole exception of the 3 mm 313ms TR protocol which only showed significant differences between the 2 conditions during the face detection. For all sequences, the amplitude differences emerge as early as 4 seconds after stimulus onset during the face detection task. Multivariate analysis: tMVPA confirmed the results observed with the univariate analysis. Importantly, tMVPA indicated a larger synchrony of multi-voxel patterns of responses for the face versus non-face conditions as early as 900 ms after stimulus onset.Conclusion
The results presented provide more evidence that the BOLD signal carries information over relatively fast time scales. The observed early latency differences across conditions suggest that ultra-fast TR protocols stand to not only improve the statistical power of fMRI, but also have the potential to offer insights into the temporal dynamics of neural processing.Acknowledgements
No acknowledgement found.References
1. Ogawa, S., Tank, D. W., Menon, R., Ellermann, J. M., Kim, S.-G., Merkle, H., & Ugurbil, K. (1992). Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. PNAS
2. Lewis, L.D., Setsompop, K., Rosen, B.R., Polimeni, J.R. (2016) Fast fMRI can detect oscillatory neural activity in humans. PNAS.
3. Vu, A.T., Phillips, J.S., Kay, K., Phillips, M.E., Johnson, M.R., Shinkareva, S.V., Tubridy, S., Millin, R., Grossman, M., Gureckis, T., Bhattacharyya, R., Yacoub, E., 2016. Using precise word timing information improves decoding accuracy in a multiband-accelerated multimodal reading experiment. Cognitive Neuropsychology.
4. Van Voorhis, S. & Hillyard, S. A. (1977). Visual evoked potentials and selective attention to points in space. Perception & Psychophysics.
5. Ramon, M., Vizioli, L., Liu-shuang, J., & Rossion, B. (2015). Neural microgenesis of personally familiar face recognition. PNAS.
Figures