0152
Functional organization of visual temporal frequency preference revealed by thalamo-visual correlation1National Institute of Mental Health, National Institutes of Health, Bethesda, MD, United States
Synopsis
Thalamo-visual connections play an important role in the visual system. Little is known about the temporal frequency tuning properties of the thalamo-visual correlation in humans. Here we demonstrated that thalamo-visual correlation is significantly modulated by the temporal frequency of a stimulus. Using correlation with thalamus as an index, human visual cortex is organized along a temporal dimension, with the anterior calcarine preferring low temporal frequencies and posterior calcarine preferring higher temporal frequencies.
Introduction
Human ability to resolve temporal variation or flicker in the brightness is limited to about 50 Hz1,2. It is natural to ask where in the system the sensitivity limitation is imposed across the multiple levels of visual processing, including thalamic and many cortical stages. The thalamus is the main source of visual information for visual cortex. The connectivity between thalamus and the occipital visual cortex can play an important role in the temporal frequency sensitivity of the visual system. In this research, we studied the temporal frequency tuning properties of the thalamo-visual correlation. In the range of 1 Hz to 40 Hz, anterior calcarine’s correlation with thalamus peaked at 14.7 Hz, while posterior calcarine’s correlation with thalamus peaked at 22.6 Hz.Methods
Twenty right-handed subjects (ten males/ten females; aged 18-50, mean age 26.5) were scanned on the Siemens 3T Prisma scanner. Visual stimulation at multiple different frequencies (1Hz, 5Hz, 10Hz, 20Hz and 40Hz) were applied through full-field (black/white) reversal of an LCD screen (BOLDscreen 32). Temporal accuracy of the LCD screen was verified independently using a photodiode. Separate runs with (i) continuous stimulation for 386 s (and a control run with no stimulation) and (ii) with a block-designed experiment (10 s ON, 20.6 s OFF). Simultaneous multi-slice (SMS) EPI was used with TR/TE = 1700/28 ms, matrix 90×90, 2.5 mm isotropic voxels, 52 slices, SMS factor = 2.
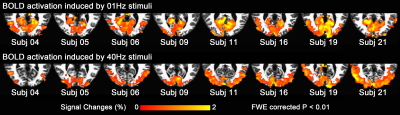
Block-design data was used to define ROIs for subsequent connectivity analyses. In particular, we used linear regression with an onset + sustained + offset BOLD response model3,4 to define two regions: (1) a calcarine region activated by 1 Hz stimuli (low-frequency ROI); (2) an occipital non-calcarine region activated by 40 Hz stimuli (high-frequency ROI).
The steady-state data was used for the connectivity analyses. For this, we take low-frequency and high-frequency ROIs as the seed regions and computed their connectivity to the whole brain. In every voxel of gray matter, we calculated its mean correlation with all voxels inside each of these two ROIs for each subject and applied group-level statistical comparison between different stimulation conditions. Then the thalamo-visual correlation vs. flickering frequencies were fitted using a Gaussian function on an ROI and voxel-wise basis. In each visual voxel, the peak frequency was extracted from the fitting curve to display if the correlation coefficient of the fitting was larger than 0.35. The frequency-dependent thalamo-visual correlation changes were also inputted to the K-means clustering algorithm to group areas according to the similarity in their frequency response properties.
Results and discussion
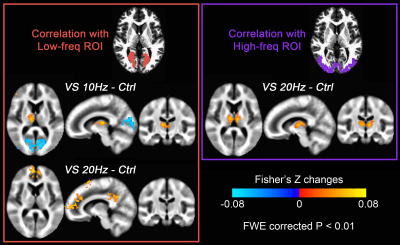
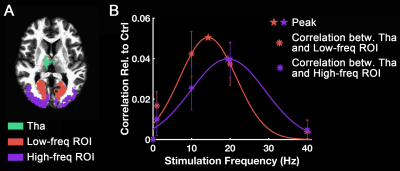
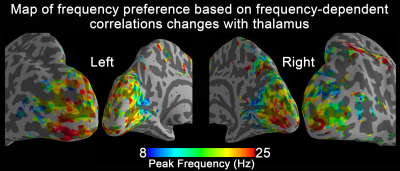
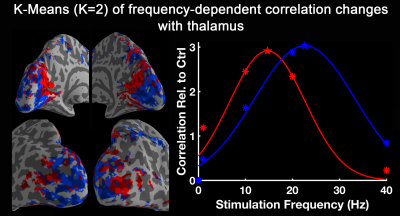
Fig. 1 shows activation maps of the sustained BOLD response for the block-designed data. It consistently shows across subjects how the 1 Hz stimuli mainly activated the calcarine region (top row), while the bottom row shows that 40 Hz stimuli mainly activated lateral occipital cortex (bottom row). These two activation areas were used to define low-frequency and high-frequency ROIs, respectively. Using the steady-state data, we computed the correlation with low-frequency and high-frequency ROIs. Compared with the control, visual stimulation at 10 Hz (VS 10Hz) significantly increased the correlation between thalamus and the low-frequency ROI, while for VS 20Hz it was the correlation between thalamus and high-frequency ROI that increased significantly (Fig. 2). The intersection of these two above-threshold sections of the thalamus was defined as the connected thalamus ROI. In Fig. 3, we report the mean correlations between connected thalamus and low-frequency/high-frequency ROIs. Both correlations were significantly modulated as a function of stimulation frequency (one-way ANOVA, p = 0.014 for correlations with low-frequency ROI, p = 0.043 for correlations with high-frequency ROI). The correlations relative to control vs. stimulation frequencies were fitted to a Gaussian function. The same fit was applied to the correlation between connected thalamus ROI and each voxel in visual areas. The voxel-wise peak frequency was displayed in Fig. 4. Using correlation with thalamus as an index, anterior calcarine prefers low temporal frequency, while posterior calcarine and lateral occipital areas prefer high temporal frequency. We also applied K-means clustering analysis to the frequency-dependent correlations and generated two clusters as in Fig. 5, with one sensitive to low frequency and the other sensitive to high frequency.Conclusions
The thalamo-visual correlation is significantly modulated as a function of temporal frequency. Using correlation with thalamus as an index, human visual cortex is organized with the anterior calcarine preferring low temporal frequencies and posterior calcarine tuned to high temporal frequencies.Acknowledgements
No acknowledgement found.References
1. Jiang Y, Zhou K, He S. Human visual cortex responds to invisible chromatic flicker. Nat Neurosci. 2007;10(5):657-662. doi:10.1038/nn1879.
2. Shady S, MacLeod DI a, Fisher HS. Adaptation from invisible flicker. Proc Natl Acad Sci U S A. 2004;101(14):5170-5173. doi:10.1073/pnas.0303452101.
3. Uludaǧ K. Transient and sustained BOLD responses to sustained visual stimulation. Magn Reson Imaging. 2008;26(7):863-869. doi:10.1016/j.mri.2008.01.049.
4. Gonzalez-Castillo J, Hoy CW, Handwerker DA, et al. Task dependence, tissue specificity, and spatial distribution of widespread activations in large single-subject functional MRI datasets at 7T. Cereb Cortex. 2015;25(12):4667-4677. doi:10.1093/cercor/bhu148.
5. Yen CCC, Fukuda M, Kim SG. BOLD responses to different temporal frequency stimuli in the lateral geniculate nucleus and visual cortex: Insights into the neural basis of fMRI. Neuroimage. 2011;58(1):82-90. doi:10.1016/j.neuroimage.2011.06.022.
Figures