0147
Imaging Primary Neuronal Activity in the Human Optical Cortex at 1.35Hz1Department of Biomedical Engineering, King's College London, London, United Kingdom, 2Brigham and Women's Hospital, Boston, MA, United States, 3Institute of Neuroradiology, University Medical Center Goettingen, Goettingen, Germany, 4MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
In this work we have developed a novel functional MRE system for humans capable of probing stiffness changes in the brain driven by monocular visual stimulation. A continuous visual stimulus was applied at an ON/OFF frequency of 1.35 Hz during a segmented 2D multi-slice MRE sequence with 3D motion encoding operating at 50 Hz vibration frequency. Significant stiffness changes were recorded between ON/OFF during the stimulus experiment that also differed in baseline to control scans (OFF/OFF). Since the BOLD signal is entirely saturated at such high stimulation frequencies, we hypothesize that stiffness changes are due to direct neuronal activities. Data match similar results obtained in mice.
Introduction
Recent studies have demonstrated the ability to perform functional MRE (fMRE) measurements in mice with higher temporal resolution than traditional BOLD fMRI that is limited by the relatively slow hemodynamic response function which is of the order of seconds1. The ability to potentially probe neuronal activities non-invasively at temporal resolutions not limited by the hemodynamic response function could provide a new tool to understand the brain circuitry. In this work, we present an fMRE system for humans capable of probing spatially localized stiffness changes in the optical cortex driven by visual stimulation. An interleaved acquisition scheme allows for paradigm switching frequencies up to 50 Hz.
Methods
Procedure: We developed a novel fMRE system (Fig.1) in which a custom-made MRE transducer2 was coupled to the head of the volunteers to induce mechanical waves at 50 Hz. The resonance spectrum of the transducer is extremely narrow-band in order to minimize data corruption from parasite frequencies. Visual stimulation was performed monocularly by a flashing light from a fiber optic light illuminator controlled by an Arduino microcontroller. We developed an interleaved multi-slice fMRE sequence on a 3T scanner [Biograph mMR, Siemens Healthcare, Erlangen, Germany] to encode wave motion while synchronizing the flashing light stimulus, the mechanical transducer and the data acquisition (Fig.2). Sequence details were: FLASH readout, flip angle = 25 deg, TE/TR: 14.8/166 ms, scan time: 4 min 20 seconds, MEG amplitude: 35 mT/m, 9 slices, 4 wave phases, GRAPPA: acceleration factor 2, 24 reference lines; FOV: 192x192 mm, 64x64 matrix and 3x3x3 mm3 resolution, 4 repetitions: 3 MEG encoding directions and a reference scan without MEG used as a phase reference. MRE measurements were performed on phantoms to characterize the reproducibility of the acquired data (Fig.3). Different paradigm switching schemes were designed to drive different visual stimuli on human subjects: (I) no stimulus in both paradigms as a control scan to characterize reproducibility of the experiment and (II) visual stimulus switching at (II) 1.35 Hz.
Data Analysis: Displacement modulated MR-phase data are pixel-wise Fourier transformed and the according amplitudes and phases at the mechanical driving frequency used to compose the complex valued displacement vector U. Each component of U is 3D Gaussian filtered (sigma=1px, support=3x3x3pixels) prior to the calculation of the curl q of U. Finally, the 3D Helmholtz wave-equation of q is solved for an isotropic wavelength via minimum χ2. Final maps of the wavelength are spike-filtered and smoothed using a 2D Gaussian filter (sigma=1px, support=3x3pixels).
Results
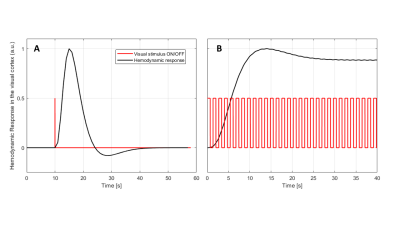
Reproducibility of the fMRE system was tested using a gel phantom: the mean reconstructed wavelength measured within a ROI - of comparable size as the one used for the human cortex analysis - showed deviations of the order or 0.02 mm, i.e. about 0.1% (Fig.3). The noise level of the human data was higher due to a reduced wave penetration originating from the presence of the cranium. Mean values of the wavelength within the visual cortex varied for human control experiments by 0.2 mm (i.e. about 1%), providing a significance threshold. Visual stimulus experiments showed significant increase in baselines stiffness within the visual cortex area of about 1 mm. Additionally, a localized increase in wavelength of 0.85 mm and 1.62 mm between OFF and ON states was measured for volunteers I and II, respectively (Fig.4). The BOLD response is entirely saturated at those switching frequencies (Fig.5). Thereby, the observed stiffness changes at 1.35 Hz between ON and OFF state localized in the visual cortex cannot be explained by changes in the hemodynamics.Discussion and Conclusion
The changes observed in viscoelastic parameters of human brain tissue when presented to switching visual stimuli are in agreement with the fMRE response measured in mice1,3. We have found an increase in stiffness when the visual stimulus was presented compared to our control, and more interestingly, we observed an additional increase in stiffness when the visual stimulus was switched off. Animal experiments using a pain paradigm and operating at 1000 Hz showed localized stiffness increase of about 1 kPa. Under the hypothesis that stiffness changes originate from cellular stiffness variations driven by ionic influx, of the order of 30 Pa, the underlying frequency power-law leads for the mice experiments to an amplification of $$$\sqrt{1000}$$$~30, in agreement with the 1 kPa changes. Here, the lower MRE driving frequency of 50 Hz would only lead to an amplification of ~7, resulting in expected wavelength changes of 1-2 mm in agreement with the observations. These results demonstrate the capability of fMRE to measure functional changes in the brain caused by neuronal activity at higher frequencies than the time resolution the hemodynamic response is able to resolve. This opens the way to perform high-temporal resolution functional studies using MRI.
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 668039.References
1. Patz S, Nazari N, Barbone PE, et al., Functional Neuroimaging in the Brain using Magnetic Resonance Elastography, In: Intl. Soc. Mag. Reson. Med. 25. Abstract 0242. Honolulu; 2017.
2. Runge J, Hoelzl S, Sudakova J, et al., A Novel MR Elastography Transducer Concept Based on a Rotational Eccentric Mass: The Gravitational Transducer. In: Intl. Soc. Mag. Reson. Med. 25. Vol 1369. Honolulu; 2017.
3. Patz S, Fovargue D, Schregel K, et al., Imaging Primary Brain Activity Using Quantitative Maps of Tissue Stiffness. Manuscript in preparation.
Figures
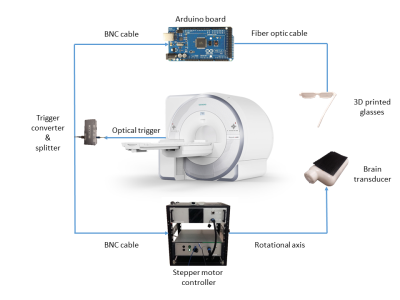
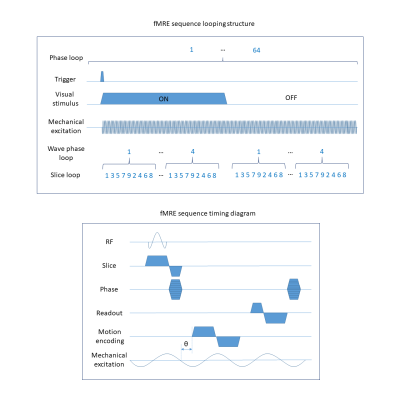
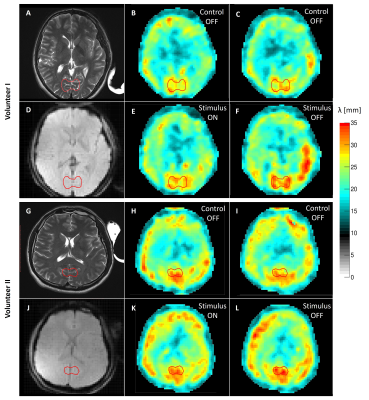
Figure 4. fMRE data in two human brains. The region of interest used in the analysis is contoured with a red line surrounding the visual cortex. A,G) T2-weighted anatomical image of the central slice shown for reference. B,H) Reconstructed wavelength map of the first paradigm acquired without visual stimulus (control scan). C,I) Reconstructed wavelength map for the second paradigm acquired without visual stimulus (control scan). D,J) fMRE magnitude image shown as an anatomical reference using the wavelength maps resolution. E,K) Reconstructed wavelength map for the first paradigm with visual stimulus switched ON. F,L) Reconstructed wavelength map for the second paradigm with visual stimulus switched OFF.