0136
Neurite Orientation Dispersion and Density Imaging: Added Value in the Detection of Tubers in Patients With Tuberous Sclerosis Complex1Department of radiology, Peking Union Medical College Hospital, Peking Union Medical College and Chinese Academy of Medical Sciences, Beijing, China, 2Department of interventional radiology, China Meitan General Hospital, Beijing, China, 3Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine, Tsinghua University, Beijing, China, 4Department of Neurosurgery, China National Clinical Research Center for Neurological Diseases, Beijing, China
Synopsis
The aim of this study was to evaluate the performance of Neurite Orientation Dispersion and Density Imaging (NODDI) in depicting cortical tubers in patients with tuberous sclerosis complex (TSC). By comparing with conventional MRI and DTI, the intracellular volume fraction (ICVF) derived from NODDI showed privilege over both techniques with higher sensitivity and better contrast ratio. Our result has revealed that NODDI was better at detecting microstructural disruption than DTI and conventional MRI sequences with a more reasonable model assumption, and may somehow shed light on the management of epilepsy in TSC patients.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant multisystem disorder with widespread central nervous system anomalies. The cerebral lesions include cortical tubers, subependymal nodules (SEN), subependymal giant cell tumors (SGCT) and white matter abnormalities. Tubers are thought to be related with epilepsy, mental retardation, and focal neurological deficits. Traditionally, MRI was used to assess the location and number of cortical tubers. Even though fluid-attenuated inversion recovery (FLAIR) was believed to be the most sensitive sequence to detect lesions, some subtle cortical tubers are still difficult to be detected. That confused clinical doctors, especially neurosurgeon, to precisely localize the responsibility lesion for seizure. Orientation Dispersion and Density Imaging (NODDI) is a newly proposed diffusion analysis technique which overcomes limitations in modeling microstructure[1]. Previous study[2] has revealed that NODDI would assist in the clinical identification and localisation of epileptogenic region in patients with focal cortical dysplasia (FCD), thus showed its promise in the management of epilepsy. So we presume that NODDI would also be helpful in depicting cortical tubers in TSC patients which are histologically comparable to FCD lesions.Methods
11 patients diagnosed with TSC were enrolled (male: female, 7:4; mean±SD age, 5.8±6.7 years). Data were collected on a MAGNETOM Skyra 3T MR scanner (Siemens Healthcare, Erlangen, Germany) with a 20-channel head-neck coil. A multi shell diffusion sequence was performed with the following parameters: TR/TE=7500/94ms; FOV=240mm; slice thickness=2.5mm; voxel size=2.0×2.0×2.5mm; 1 non-diffusion weighted acquisition, 30 directions with b-value 1000 s/mm2, 30 directions with b-value 2000 s/mm2, total scan time 8 minutes. The DTI and conventional MRI sequences including axial T1WI (T1-weighted image), axial T2WI (T2-weighted image) and axial FLAIR-T2 images were also performed. The NODDI data were postprocessed with the NODDI Matlab Toolbox to generate maps of intracellular volume fraction (ICVF) and orientation dispersion index (ODI). We evaluated the imaging features of each modality and counted the numbers of cortical tubers on each sequence. The relative comparison of ICVF versus T1, T2 and FLAIR-T2, respectively, was expressed as a gain or loss in the number of detected tubers. The statistical analysis was performed patient-wise with paired t-test or Wilcoxon test according to whether the difference values obeyed a normal distribution or not. P values <0.05 were considered as statistically significant.Results
For 10 patients, areas of reduced ICVF were clearly identified and co-located with the abnormality on conventional MRI (except for patient 4 who was characterized by white matter abnormality while no cortical tubers were detected on each sequence). Lesions observed on ICVF tend to be more clearly defined and have a higher contrast ratio compared with FLAIR and other sequences (see figure 1). The changes of ODI were not apparent. Fractional anisotropy (FA) derived from DTI also failed to detect tubers. On the contrary, ICVF was proved to be able to detect lesions even invisible on FLAIR image (see figure 2). The statistical analysis demonstrated that the number of tubers on ICVF images was higher than those on T1WI (p=0.003, paired t-test), T2WI (p=0.008, Wilcoxon test) and FLAIR images (p=0.02, Wilcoxon test). The detailed results are listed in table 1.Discussion and conclusion
To the best of our knowledge, this is the first study to evaluate the application value of NODDI in TSC patients with a clinically feasible scan protocol of 8 minutes. Our results have indicated that ICVF derived from NODDI outperformed both the conventional MRI and DTI in depicting the cortical tubers. NODDI assumes three diffusion compartments in each voxel: cerebrospinal fluid (CSF), extraneurite and intraneurite. So it was enabled to distinguish two key factors contributing to changes in FA-- neurite density (ICVF) and fibre orientation dispersion (ODI), and minimize the partial volume effect of CSF. Therefore, it may be more sensitive with the changes in tissue microstructure in both grey and white matter and may potentially assist in the identification of lesions of TSC patients which was characterized by the disruption of normal cortical architecture. What’s more, holding the quality of sensitively detecting and clearly delineating the lesions, NODDI might probably be able to recognize epileptogenic tubers and determine the margins of the epileptogenic regions. Which is really important for TSC patients, as epilepsy was reported to affect 90% of these patients with 30% turned out to be intractable. However, further studies with ROI analysis are needed to validate these assumptions.Acknowledgements
No acknowledgement found.References
[1] Zhang H,Schneider T,Wheeler-Kingshott CA, et al. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage, 2012, 61(4): 1000-1016.
[2] Winston GP,Micallef C,Symms MR, et al. Advanced diffusion imaging sequences could aid assessing patients with focal cortical dysplasia and epilepsy. Epilepsy Res, 2014, 108(2): 336-339.
Figures
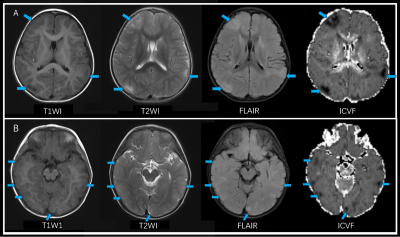
Figure 1: Lesions more remarkable on ICVF. A 2-year old boy presented with epilepsy. Tubers are more clearly defined and presented with a higher contrast ratio on ICVF than T1WI, T2WI and FLAIR (blue arrows).
Note: T1WI, T1-weighted image; T2WI, T2-weighted image; FLAIR, fluid-attenuated inversion recovery; ICVF, intracellular volume fraction.
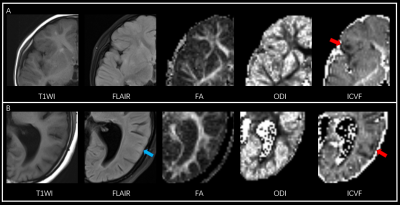
Figure 2: Lesions only visible on ICVF. A. 2-year old boy presented with epilepsy, a tuber on right frontal lobe was clearly demonstrated on ICVF (red arrow) while other sequence failed to show. B. 15-year old girl presented with epilepsy, a tuber on the left temporal lobe was depicted on ICVF (red arrow), the lesion seems rather obscure with slightly increased signal intensity on FLAIR (blue arrow), and was initially thought to be invisible.
Note: T1WI, T1-weighted image; FLAIR, fluid-attenuated inversion recovery; FA, fractional anisotropy; ODI, orientation dispersion index; ICVF, intracellular volume fraction.
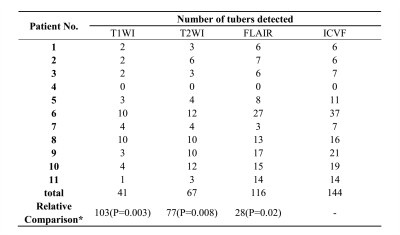
Table 1:Analysis of tuber count and relative comparisons of ICVF versus the T1, T2 and FLAIR images.
Note: T1WI, T1-weighted image; T2WI, T2-weighted image; FLAIR, fluid-attenuated inversion recovery; ICVF, intracellular volume fraction.
*Relative Comparison refers to a total gain in the number of detected tubers on ICVF compared with other modalities. P value was obtained from the patient-wise analysis by paired t-test for the difference between ICVF and T1WI, and by Wilcoxon analysis for the difference between ICVF and T2WI as well as FLAIR.