0134
Microscopic diffusion anisotropy reveals microstructural heterogeneity of malformations of cortical development associated with epilepsy: A b-tensor encoding study at 7T1Clinical Sciences Lund, Medical Radiation Physics, Lund University, Lund, Sweden, 2Clinical Sciences Lund, Neurology, Lund University, Lund, Sweden, 3Random Walk Imaging AB, Lund, Sweden, 4Clinical Sciences Lund, Diagnostic Radiology, Lund University, Lund, Sweden, 5Skane University Hospital, Department of Clinical Sciences Lund, Neurology, Lund University, Lund, Sweden, 6Skane University Hospital, Department of Clinical Sciences Lund, AKVH-Neurology Helsingborg, Lund University, Lund, Sweden
Synopsis
Malformations of cortical development are macro- or microscopic abnormalities of the cerebral cortex. Here, we investigated such malformations associated with epilepsy using b-tensor encoding, which is a recently developed technique that permits estimation of microscopic anisotropy also in regions where diffusion is isotropic on the voxel level. Results show a large heterogeneity in microscopic anisotropy between lesions, which we hypothesize represents different levels of axonal content. The characteristics of some types of lesions depended strongly on whether they were associated to other lesions, which could be clinically helpful for indicating hidden sources of epileptic seizures.
Introduction
Malformations of cortical development associated with epilepsy is a diverse group of gray matter pathologies. Here we study two main types: focal cortical dysplasia, which involves abnormality of cortical structure and neuronal morphology, and cortical heterotopia, which involves ‘misplaced neurons’ due to abnormal migration1. Precise characterization of these lesions using conventional MRI is complicated by the presence of different histopathological subtypes and of other associated malformations2. Here we investigate whether an improved characterization can be gained by b-tensor encoding, which is a recently developed concept in diffusion-MRI that allows detection of microscopic anisotropy also in tissue that is nearly isotropic on the voxel level, such as gray matter. Results show variable levels of microscopic anisotropy within lesions, a feature not detectable with conventional diffusion encoding techniques3.Methods
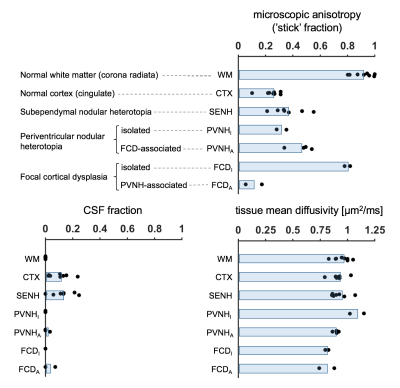
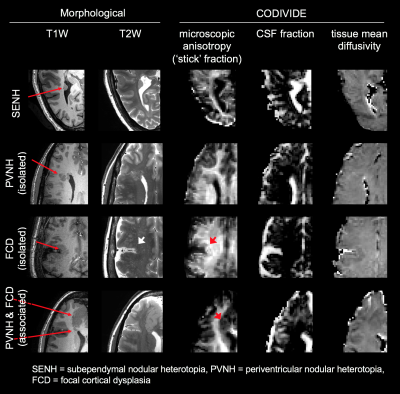
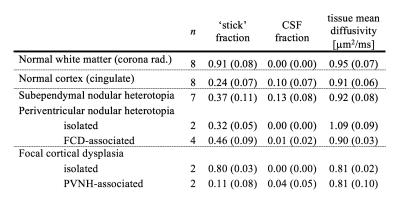
Eight subjects with malformations of cortical development associated with epilepsy (age 35±12, 6 females) underwent examination on a 7T MRI system (Achieva, Philips, Best, The Netherlands), after informed consent had been obtained. A prototype diffusion sequence was used to acquire data with both linear and spherical tensor encoding4-5. The acquisition used TR/TE=3500/89 ms/ms at 2×2×4 mm3 resolution and b=[0 0.1 0.5 1.0 1.5 2.0] ms/μm2 distributed over up to 12 directions. In addition, T1W images were acquired using a 3D TFE sequence (TE/TR=2.8/8 ms/ms, 0.6 mm3 isotropic) and T2W images were acquired using a TSE sequence (TE/TR=60/3500 ms/ms, 0.5×0.5×0.75 mm3). Diffusion data were analyzed with the CODIVIDE method to estimate the level of microscopic anisotropy (‘stick’ fraction), the signal fraction of CSF and the mean diffusivity of tissue3. Data were ‘powder-averaged’ across encoding directions before fitting to obtain an orientation invariant signal4. The estimated parameters were extracted from regions of interest (ROIs) in normal white matter (WM; corona radiata), normal cortical gray matter (CTX; cingulate) and seventeen lesions. The lesions were identified and classified by a neuroradiologist as seven subependymal nodular heterotopia (SENH), six periventricular nodular heterotopia (PVNH) and four focal cortical dysplasia (FCD). Among the PVNH, two were isolated and four were FCD-associated. Among the FCD, two were isolated and two were PVNH-associated.Results
The lesions exhibited very different levels of microscopic anisotropy (Table 1, Fig. 1 and 2). The cortical heterotopias (SENH and both forms of PVNH) exhibited slightly higher microscopic anisotropy than the normal cortex. Meanwhile, the level of microscopic anisotropy was exceptionally high in isolated FCD and close to zero in PVNH-associated FCD. While most lesions had T1W and T2W intensities similar to the cortex, isolated FCD showed ‘gray-white matter blurring’ and a reduced intensity on T2W images (Fig. 2). The tissue mean diffusivity was similar to in the normal cortex in the heterotopias but lower in FCD, indicating a higher cellularity. The CSF fraction was generally below 10%.Discussion
We used b-tensor encoding to demonstrate a strong heterogeneity in the level of microscopic anisotropy among malformations of cortical development associated with epilepsy. We hypothesize that this finding reflects a variable axonal content in the lesions, since previous results indicate that dendrites contribute negligibly to microscopic anisotropy6. This hypothesis suggests high levels of axons in isolated FCD lesions, presumably myelinated, which would be consistent with their hypointense appearances on T2W images. The low levels of anisotropy in the PVNH-associated FCD could indicate the presence of so-called ‘balloon cells’, which lack synaptic contacts7. The second lesion type, cortical heterotopia, is known to feature immature neurons with limited structural connectivity8. It was thus surprising to find levels of microscopic anisotropy higher than in the normal-appearing cortex. This could possibly be due to contamination of white matter in the ROIs.Conclusions
We demonstrate that b-tensor encoding yields an improved characterization of the tissue microstructure in malformations of cortical development associated with epilepsy. Definite conclusions require larger sample sizes, as well as further studies to investigate the axonal content in these lesions by histology. The potential ability to differentiate between isolated and PVNH-associated focal cortical dysplasia should be explored further, since it could be clinically helpful by indicating hidden (associated) sources of epileptic seizures.Acknowledgements
We thank Philips for providing access to the pulse programming environment.References
1. Cendes, F., et al., Neuroimaging of epilepsy. Handbook of clinical neurology, 2016. 136: p. 985.
2. Blümcke, I., et al., The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia, 2011. 52(1): p. 158-174.
3. Lampinen, B., et al., Neurite density imaging versus imaging of microscopic anisotropy in diffusion MRI: A model comparison using spherical tensor encoding. NeuroImage, 2017. 147: p. 517-531.
4. Lasič, S., et al., Microanisotropy imaging: quantification of microscopic diffusion anisotropy and orientational order parameter by diffusion MRI with magic-angle spinning of the q-vector. Frontiers in Physics, 2014. 2: p. 11.
5. Westin, C.-F., et al., Q-space trajectory imaging for multidimensional diffusion MRI of the human brain. NeuroImage, 2016. 135: p. 345-362.
6. Lampinen, B., et al., Microscopic Anisotropy in gray matter is evidence of myelinated axons, but not dendrites? An in vivo study using diffusion MRI with variable shape of the b-tensor. In proceedings for the 25th Annual Meeting of ISMRM, Honolulu, Hawaii, USA, 2017. p. 716. 2017.
7. Alonso-Nanclares, L., et al., Microanatomy of the dysplastic neocortex from epileptic patients. Brain, 2004. 128(1): p. 158-173.
8. Hannan, A.J., et al., Characterization of nodular neuronal heterotopia in children. Brain, 1999. 122(2): p. 219-238.
Figures