0128
In vivo cancer detection and dynamics with magnetic particle imaging1Department of Bioengineering, University of California, Berkeley, CA, United States, 2Lodespin Labs, Seattle, WA, United States, 3Department of Material Science and Engineering, University of Washington, Seattle, WA, United States, 4Magnetic Insight, Inc., Alameda, CA, United States, 5Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA, United States
Synopsis
Magnetic Particle Imaging (MPI) is a novel, high-contrast, and quantitative imaging modality that directly detects superparamagnetic iron oxide nanoparticle (SPIO) tracers. These SPIOs have been previously used as a MRI contrast agent. However, with MRI SPIOs are limited by poor specificity and difficulty associated with quantifying the negative signal. MPI enables the direct detection of these SPIOs with both high sensitivity and positive contrast. MPI is well poised to support MRI in developing a clinically translatable cancer detection platform with SPIO. Here we demonstrate in vivo cancer detection and dynamic perfusion imaging with MPI.
Introduction:
Magnetic Particle Imaging (MPI) is a novel, high-contrast, and quantitative imaging modality with zero depth attenuation and exquisite tracer sensitivity.1,2,3 The system uses magnetic field gradients to directly detect the intense electronic magnetization of superparamagnetic iron oxide nanoparticles (SPIOs), unlike the much weaker nuclear paramagnetism of water detected in MRI. SPIOs have been previously used as a MRI contrast agent producing a negative image contrast. They do not emit ionizing radiation and have strong biocompatibility; with FDA approved SPIO tracers available even for patients with Chronic Kidney Disease.4 However, with MRI SPIOs are limited by poor specificity and difficulty associated with quantifying the negative signal. Due to its direct detection, high sensitivity and positive contrast, MPI is uniquely poised as a clinically translatable cancer detection platform. Here we demonstrate in vivo cancer imaging with MPI.5Methods:
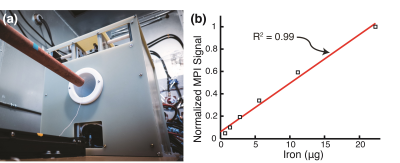
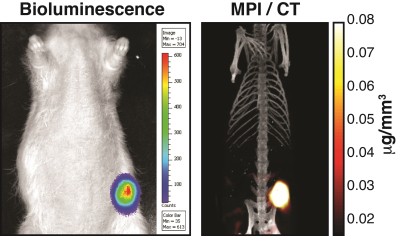
Two cohorts of athymic nude rats (Groups A & B) were injected with 7 million MDA-MB-231-luc tumor cells subcutaneously and monitored for 4 weeks. The animals were injected with long circulating MPI-tailored SPIOs (LS-008, Lodespin Labs). In Group A (n=3), the tumors were implanted in the left lower mammary fat pad and LS-008 was intravenously administered at a dose of 15 mg/kg. In Group B (n=3), the tumors were implanted at the right lower flank and LS-008 was intravenously administered at a dose of 5 mg/kg. The animals were then scanned at multiple time-points post-injection: 10 and 30 minutes, 1, 2, 4, 6, 24, 48 and 96 hours. A Field Free Point (FFP) MPI scanner was used (Resolution: 1.2 mm, Group A FOV: 4 cm × 4 cm × 8.5 cm (cropped 5.8 cm), Scan Time: 5 minutes; Group B FOV: 4 cm × 4 cm × 14.5 cm, Scan Time: 9 minutes). MATLAB is used with a National Instruments DAQ module for signal generation, acquisition, and image reconstruction. See Figure 1a for a photo of the custom FFP MPI scanner. MPI allows for accurate quantitiation due to the lineararity of the signal with iron concentration (Figure 1b) For anatomical reference, micro-CT (General Electric EVS RS-09) scans of two of the animals were acquired (Resolution: 93μm, FOV: of 4 cm × 4.7 cm × 16.5 cm, Scan Time: 25 minutes). To confirm the presence of the tumor, the rats were injected with Luciferin for Bioluminescence imaging.Results:
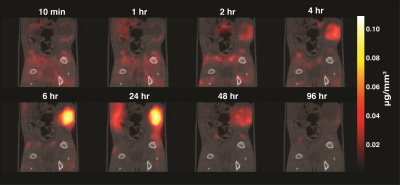
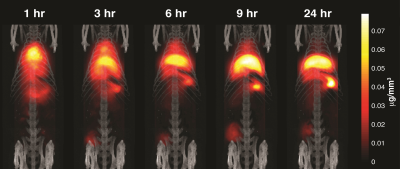
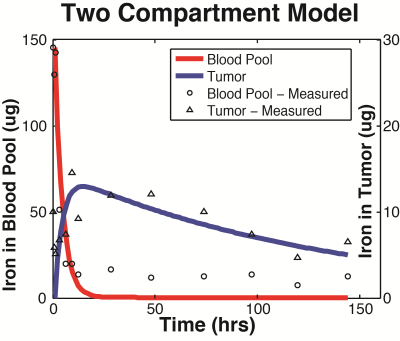
Group A tumors were highlighted with a peak tumor-to-background ratio of roughly 50-to-1 seen in Figure 2. The tumor perfusion dynamics are well appreciated; with initial wash-in on the tumor rim, peak uptake at 6 hours, and washout beyond 48 hours (Figure 3). Group B tumors were injected with a lower dose of tracer and the whole body dynamic distribution was captured via MPI over time (Figure 4). The injected nanoparticles are first distributed uniformly in the vascular system, with large blood volume organs such as the heart and lungs and large blood vessels distinguishable. The iron-oxide nanoparticles are subsequently cleared from the blood by the RES, highlighting the liver and spleen. Simultaneously, the contrast and sensitivity inherent to MPI allows for the tumor to be clearly visible through time. The particles used in this study had no active targeting moieties and were systemically administered. The contrast allows clear visualization of the tumor. In addition, MPI enables quantitative analysis of tracer dynamics – blood pool and tumor iron content for Group B rats were successfully fit to a two compartment model as shown in Figure 5.Discussion and Conclusions:
Our preliminary results demonstrate the potential of MPI as a sensitive and quantitative cancer imaging platform. Even with passive particle uptake, we were able to see a superb tumor-to-background signal ratio of ~50. The particles were likely retained within the tumor due to the enhanced permeability and retention (EPR) effect.6 The high sensitivity of and superb contrast inherent to MPI allows for dose-limited sensitivity approaching that of PET and SPECT. With continual development, we anticipate MPI to emerge as a robust imaging platform for evaluating cancer targeting moieties, tumor perfusion, detecting small metastases, and monitoring cell migration. Acknowledgements
The authors would like to acknowledge funding support from NIH 5R01EB019458-03, NIH5R24MH106053-03, UC Discovery Grant 29623, W. M. Keck Foundation Grant 009323, andNSF GRFP for this work. Additionally, work at Lodespin Labs and University of Washington was supported by NIH 1R41EB013520-01 and NIH 2R42EB013520-02A1.References
1. Gleich and Weizenecker. Tomographic imaging using the nonlinear response of magnetic particles. Nature. 435, 1214-1217. (2005)
2.Goodwill and Conolly. The X-space formulation of the magnetic particle imaging process. IEEE TMI. 29(11):1851-9 (2010)
3. Zheng et al. Magnetic Particle Imaging tracks the long-term fate of in vivo neural cell implants with high image contrast. Scientific Reports, 5:14055. (2015)
4. Lu et al. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J. Hematol. 85(5):315-9. (2010).
5. Yu et al. Magnetic Particle Imaging: A novel in vivo imaging platform for cancer detection. Nano Letters. 17(3): 1648-1654. (2017)
6. Maeda, J et al. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 1;65(1-2):271-84 (2000).
Figures