0124
Assessment of Distant Tumor Stimulation from Liver Radiofrequency Ablation in a Rat Breast Carcinoma Model using Hyperpolarized 13C-Pyruvate MRI1Radiology, Beth Israel Deaconess Medical Center, Boston, MA, United States
Synopsis
Radiofrequency ablation (RFA) is commonly used to treat tumors such as hepatocellular carcinomas. However, there is evidence that liver RFA can stimulate growth in tumors at other sites, a process attributed to the HFG/c-Met pathway. Hyperpolarized 13C-pyruvate MRI showed increased intratumoral lactate production in subcutaneously-implanted R3230 tumors following RFA of normal liver in a rat model. This effect is suppressed when liver RFA is combined with a c-Met inhibitor. 13C MRI could potentially be used to identify tumors at risk for this off-target effect.
Introduction
Radiofrequency ablation (RFA) is used to treat hepatocellular carcinoma (HCC), liver metastases, and other tumors.1-3 However, there is increasing evidence that off-target RFA effects may include induction of distant tumor growth.3–10 One identified mediator of this process is the hepatocyte growth factor (HGF)/c-Met signaling axis, and adjuvant c-Met inhibition has been shown to suppress distant tumor growth following hepatic RFA.10,11 This has both direct and indirect effects on tumor glycolysis, which can be interrogated with hyperpolarized 13C-pyruvate MRI (h13C-pyruvate MRI), offering a potential clinical method to detect at-risk lesions.12,13 Here we use h13C-pyruvate MRI to track changes in lactate production in a metastatic breast carcinoma model stimulated by off-target liver RFA.Methods
Female Fisher rats (120-150 g) were implanted with R3230 rat breast adenocarcinoma cells (107 cells / 300 μL injection) in the mammary fat pad subcutaneously. Tumor size was tracked daily using calipers. When tumors reached 10-12 mm in average diameter (Day 0), animals were assigned to one of three study arms: sham surgery (laparotomy but no treatment, n = 9), hepatic RFA (21-guage electrode placed on the surface of the right hepatic lobe and energized to 70 ± 2°C for 5 minutes, n = 13), or hepatic RFA + adjuvant c-Met inhibition (PHA-665752, single dose IP, 0.83 mg/kg, administered 24 hours post-RFA, n= 2).
MRI was performed on at 9.4 T horizontal-bore small-animal system (Biospec, Bruker). h13C-pyruvate MRI was performed using an echo planar spectroscopic imaging (EPSI) sequence with FOV 6 cm, 16 x 16 matrix, 3 mm slice thickness. ~120 mMol of h13C-pyruvate was injected into animals via the tail vein. Imaging was performed 24 hours prior to treatment and 72 hours post-treatment.
Image processing was performed in Mathematica (Wolfram) and ImageJ (NIH). Lactate production was reported as the lactate:pyruvate ratio (LPR), obtained from the average from the three voxels demonstrating the highest lactate signal within each tumor.
Results
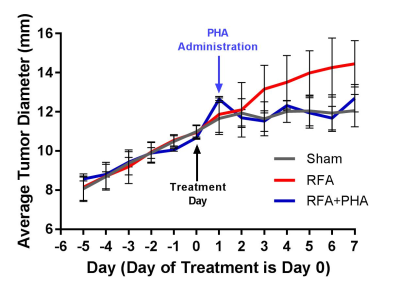
Growth curves for the three study arms are shown in Fig 1. Hepatic RFA alone resulted in increased growth of the distant R3230 tumor compared to sham treatment (0.50 ± 0.13 mm/day and 0.11 ± 0.07 mm/day, respectively, p<0.0001). In the hepatic RFA + adjuvant c-Met inhibition group (RFA+PHA), tumor growth accelerated in the initial 24 hours after RFA and prior to PHA administration, but declined following PHA administration, with a post-treatment growth rate (0.13 ± 0.09 mm/day) similar to the sham group (p=0.58).
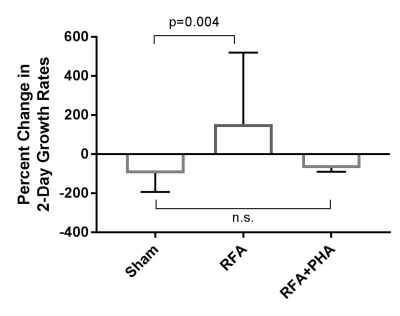
Stimulated growth in the hepatic RFA arm relative to sham was also seen when growth was measured across a 48h span at the two imaging timepoints (p=0.004), but there was no difference between sham and RFA+PHA arms (Fig 2).
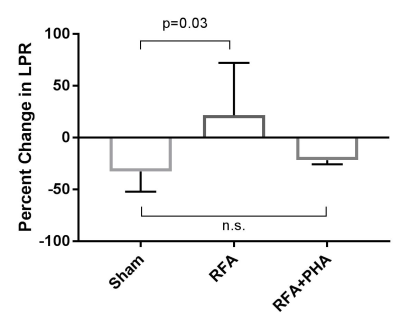
LPR was elevated in the hepatic RFA arm relative to the sham arm (p=0.03), and there was no statistically significant difference between the sham and RFA+PHA arm (Fig 3).
Discussion
In this study, we used in vivo hyperpolarized 13C-pyruvate MRI to demonstrate that liver RFA results in an increase in glycolysis and lactate production within a distal R3230 tumor, and that this effect correlates with an increase in tumor growth rate. Furthermore, the stimulation in both growth and glycolysis is suppressed by c-Met inhibition. There is close coupling between angiogenesis, hypoxia, and glycolysis via signaling pathways downstream from c-Met, most notably HIF-1⍺ and PI3K, which induce both vascular proliferation and metabolism. We attribute these to the observed increase in tumor glycolysis in the hepatic RFA arm in our study. Additional studies are needed to further elucidate the degree of coupling between HIF-1⍺ and PI3K to lactate production. The results so far highlight the potential for h13C-pyruvate MRI as a potential in vivo tool to detect lesions at risk for off-target RFA stimulation and to help identify additional potential therapeutic targets for multiple tumor types.Conclusion
In vivo h13C-pyruvate MRI shows increased lactate production within a metastatic breast tumor model following RFA of normal liver, and may potentially be used to identify lesions at risk for this behavior. The MRI results correlate with stimulation in tumor growth, a phenomenon that has also been observed clinically. This method could potentially offer a new diagnostic tool in the management of patients undergoing thermal ablation therapies.Acknowledgements
No acknowledgement found.References
1. Donadon, M. et al. Hepatocellular Carcinoma: The Role of Interventional Oncology. Liver Cancer 6, 34–43 (2017).
2. de Baère, T. et al. Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann. Oncol. 26, 987–991 (2015).
3. Lencioni, R. et al. Early-Stage Hepatocellular Carcinoma in Patients with Cirrhosis: Long-term Results of Percutaneous Image-guided Radiofrequency Ablation. Radiology 234, 961–967 (2005).
4. Kang, T. W. et al. Small Hepatocellular Carcinoma: Radiofrequency Ablation versus Nonanatomic Resection—Propensity Score Analyses of Long-term Outcomes. Radiology 275, 908–919 (2015).
5. Mittal, S. & El-Serag, H. B. Epidemiology of HCC: Consider the Population. J. Clin. Gastroenterol. 47, S2–S6 (2013).
6. Kang, T. W. et al. Aggressive Intrasegmental Recurrence of Hepatocellular Carcinoma after Radiofrequency Ablation: Risk Factors and Clinical Significance. Radiology 276, 274–285 (2015).
7. Tanis, E. et al. Local recurrence rates after radiofrequency ablation or resection of colorectal liver metastases. Analysis of the European Organisation for Research and Treatment of Cancer #40004 and #40983. Eur. J. Cancer 50, 912–919 (2014).
8. N’Kontchou, G. et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology 50, 1475–1483 (2009).
9. Lee, D. H. et al. Radiofrequency Ablation for Intrahepatic Recurrent Hepatocellular Carcinoma: Long-Term Results and Prognostic Factors in 168 Patients with Cirrhosis. Cardiovasc. Intervent. Radiol. 37, 705–715 (2014).
10. Ahmed, M. et al. Hepatic Radiofrequency Ablation–induced Stimulation of Distant Tumor Growth Is Suppressed by c-Met Inhibition. Radiology 279, 103–117 (2015).
11. Rozenblum, N. et al. Oncogenesis: An ‘Off-Target’ Effect of Radiofrequency Ablation. Radiology 276, 426–432 (2015).
12. Brindle, K. M., Bohndiek, S. E., Gallagher, F. A. & Kettunen, M. I. Tumor imaging using hyperpolarized 13C magnetic resonance spectroscopy. Magn. Reson. Med. 66, 505–519 (2011).
13. Keshari, K. R. et al. Metabolic Reprogramming and Validation of Hyperpolarized 13C Lactate as a Prostate Cancer Biomarker Using a Human Prostate Tissue Slice Culture Bioreactor. The Prostate 73, 1171–1181 (2013).
Figures