0123
Oxygen enhanced-MRI detects radiotherapy-induced change in hypoxia in xenograft models and lung cancer patients1Division of Cancer Sciences, University of Manchester, Manchester, United Kingdom, 2Department of Clinical Oncology, The Christie Hospital NHS Trust, Manchester, United Kingdom, 3Division of Informatics, Imaging and Data Sciences, University of Manchester, Manchester, United Kingdom, 4Division of Pharmacy and Optometry, University of Manchester, Manchester, United Kingdom, 5Bioxydyn Ltd, Manchester, United Kingdom, 6Department of Radiology, The Christie Hospital NHS Trust, Manchester, United Kingdom
Synopsis
Oxygen-enhanced MRI (OE-MRI) has shown promise as a technique for quantifying and spatially mapping tumour hypoxia. Here, we report a world first-in-man study showing that OE-MRI signals in perfused tumour can non-invasively track changes in hypoxia induced by radiotherapy. We show that OE-MRI detects (1) reduction in hypoxia in Calu6 xenografts and that this change is due to hypoxia modification; and (2) reduction in hypoxia is also seen in 14 patients with non-small cell lung cancer. These data support first-in-man use of OE-MRI biomarkers in clinical trials of (chemo)-radiotherapy as single agent or in combination with hypoxia-modifying agents.
Introduction
Tumour hypoxia is a feature of most solid tumours 1. Furthermore, persistence of hypoxia after initial (chemo)-radiotherapy is a poor prognostic indicator 2. There is, therefore, need to develop non-invasive imaging biomarkers of hypoxia that identify and map hypoxia before, during and after therapy. Oxygen enhanced MRI (OE-MRI) is an emerging imaging technique for quantifying and spatially mapping tumour hypoxia in vivo. Previously, we combined OE-MRI and gadolinium-based dynamic contrast-enhanced MRI (DCE-MRI) to derive a voxel-wise signature of hypoxia and perfusion, that has been validated against pathology in multiple xenograft models of cancer 3. Here, we report a world first-in-man study that shows that MRI can identify and spatially map changes in tumour oxygenation induced by radiotherapy. Initial evidence was derived from a preclinical lung cancer model (Calu6) which was accompanied by pathology validation. Then comparable data were found in patients with non-small cell lung cancer (NSCLC).Methods
Preclinical study
Studies complied with UK guidelines on animal welfare. Tumours were propagated by injecting 0.1 ml of Calu6 cells (2x107 cells/ml) intra-dermally on nude mice. When tumours were >200mm3 (callipers), mice were anaesthetized (2% isoflurane carried in 21% oxygen). Core temperature was controlled at 36°C.
Imaging was on a 7T Magnex instrument interfaced to a Bruker Avance III system, using a volume transceiver coil. Mice underwent MRI (day 0) and then received a single 10Gy fraction of radiotherapy (treated; n= 9) or sham (control; n=9) after the first scan. Subsequent imaging was at days 3 and 10. Both OE-MRI and DCE-MRI used variable flip angle (VFA) spoiled gradient echo (SPGR) acquisitions to calculate native tissue T1, followed by dynamic sequences to quantify change in longitudinal relaxation rate (ΔR1) 4. For OE-MRI, an oxygen-challenge was performed switching from medical air (21% oxygen) to 100% oxygen. For DCE-MRI, Gd-DOTA injected into a tail vein. Pathology was obtained in a separate Calu6 cohort at day 10.
Clinical study
NSCLC patients with stage I-IV disease were recruited prospectively following research ethics approval. All patients provided written informed consent. MRI was performed in 14 patients before radiotherapy and then after approximately 15 fractions of (chemo)-radiotherapy. Patients breathed medical air followed by 100% oxygen in an experimental design that paralleled the preclinical study.
Image analysis
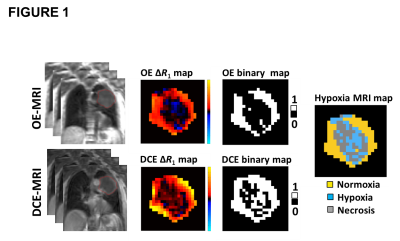
Non-perfused voxels were identified as IAUC60 ≤ 0 mmol.s. Subsequent OE-MRI analysis was restricted to perfused tumour (figure 1). Oxygen-enhancing (Oxy-E) voxels were defined by significant increase in the R1 values of dynamic time points acquired on 100% oxygen breathing compared with the R1 in baseline time points (using t-tests). Voxels without significant oxygen enhancement were termed oxygen refractory (Oxy-R). Previous studies validated Oxy-R as a biomarker of tumour hypoxia and Oxy-E as a biomarker of normoxic tumour 3.
Results
Preclinical study
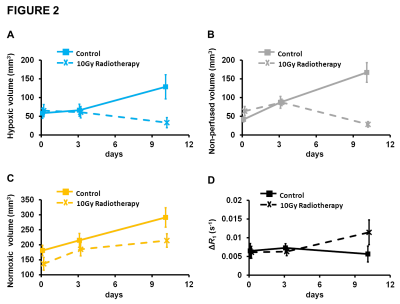
Calu6 tumours receiving radiotherapy showed reduction in hypoxic tumour volume at day 10 – measured by perfused Oxy-R – relative to control (p=0.029; figure 2A). This was accompanied by reduction in non-perfused tumour volume (p<0.001; figure 2B). The normoxic tumour volume, measured by perfused Oxy-E, was unchanged relative to control (figure 2C). Notably, median oxygen-enhanced ΔR1 increased in treated tumours but only with borderline significance (figure 2D). In a separate Calu6 xenograft cohort, immunohistochemistry showed reduction in hypoxia at day10.
Clinical study
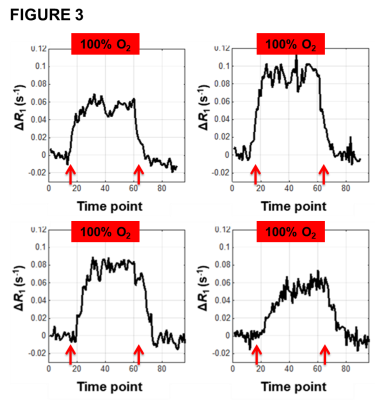
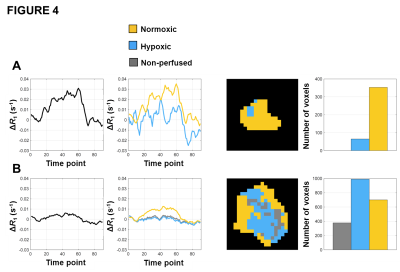
No adverse events were reported or detected on clinical examination in any of the 14 patients. Oxygen challenge resulted in significant increase in measured R1 in the aorta in all patients (p<0.0001). Signal changes reverted to baseline once the oxygen challenge was removed and air breathing resumed (figure 3). Oxygen challenge resulted in significant increase in overall tumour R1 in 83% of tumours. However, all tumours demonstrated intra-lesion spatial heterogeneity in oxygen-induced ΔR1; when combined OE and DCE analyses were used, all tumours showed spatially coherent regions of oxygen-enhancement, hypoxia and necrosis (figure 4; data for a small tumour (A) and a large tumour (B)).
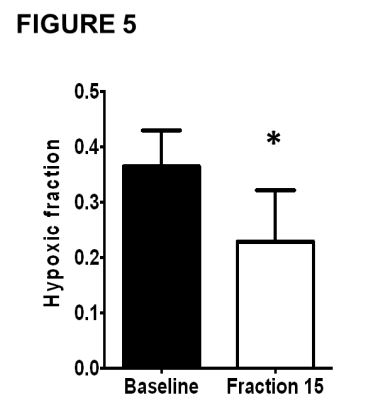
Ten patients had tumours with measurable hypoxia – defined as hypoxic fraction (measured by perfused Oxy-R) >5% – on pre-treatment MRI. The hypoxic tumour volume decreased at mid-treatment compared to baseline (p=0.0150; figure 5), mirroring results seen in Calu6 tumours. The lesions without hypoxia on baseline MRI did not develop any hypoxia at mid-treatment.
Conclusion
These data provide biological validation 5 and support first-in-man use of dynamic OE-MRI biomarkers of hypoxia in clinical trials of (chemo)-radiotherapy in combination with hypoxia-modifying agents.Acknowledgements
Cancer Research UK (CRUK) Clinician Scientist award (grant C19221/A22746) to J. P. B. O’Connor and CRUK and EPSRC Cancer Imaging Centre in Cambridge and Manchester funding to The University of Manchester (grant C8742/A18097) to G. J. M. Parker and J. P. B. O’ConnorReferences
1 Wilson WR et al. Targeting hypoxia in cancer therapy. Nat Rev Cancer 2011; 11: 393-410.
2 Zips D et al. Exploratory prospective trial of hypoxia-specific PET imaging during radiochemotherapy in patients with locally advanced head-and-neck cancer. Radiother Oncol 2012; 105:21-28.
3 O'Connor JPB et al. Oxygen-Enhanced MRI Accurately Identifies, Quantifies, and Maps Tumor Hypoxia in Preclinical Cancer Models. Cancer Res 2016; 76: 787-95.
4 Little RA et al. In vivo OE-MRI quantification and mapping of response to hypoxia modifying drugs Banoxantrone and Atovaquone in Calu6 xenografts. Proceedings ISMRM 2016; 25: 2919.
5 O’Connor JPB et al. Imaging Biomarker Roadmap for Cancer Studies. Nat Rev Clin Oncol 2017; 14: 169-186.
Figures