0118
Online Super-resolution 4D T2-weighted MRI for MRI-guided Radiotherapy1Joint Department of Physics, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom, 2CR-UK Cancer Imaging Centre, The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, London, United Kingdom
Synopsis
To assist treatment delivery to moving tissues on hybrid MRI-guided radiotherapy systems, high quality midposition (of the respiratory cycle) and 4D-T2w images are desirable. Current approaches to rapidly obtaining 4D-T2w MRI are often limited by thick slices, incomplete motion information and binning artefacts. Midposition and 4D-T2w images were calculated using motion-modelling and a super-resolution reconstruction, and verified by comparison with the initially acquired images. Calculated 4D-T2w images exhibited high spatiotemporal resolution (1.0x1.0x1.0 mm3, 8 respiratory phases), displayed reduced binning artefacts and no missing data. An acquisition time of 5.0-7.5 minutes was found sufficient to obtain representative midposition T2w images.
Purpose
Midposition (time-weighted mean position of the respiratory cycle) (MidP) T2w and 4D-T2w images enable optimised treatment delivery to moving tissues on MRI-guided radiotherapy (MRIgRT) systems1. Existing methods2 that retrospectively sort dynamic 2D-T2w MRI, often suffer from large slice-thickness, discontinuities between slices (stitching artefacts) and data incompleteness, if slices were not acquired during all respiratory phases. Here, a motion-modelling and super-resolution reconstruction (SRR) method is devised, verified against ground-truth data and shortened acquisitions are investigated.Methods
Eight volunteers were scanned in free breathing at 1.5 T (MAGNETOM Aera, Siemens Healthcare) with a 2D-T2w HASTE sequence in both sagittal and axial orientation (voxel-size 1.5x1.5x5 mm3, TE 64 msec, effective TR per slice 13.6-14.2 sec, in-plane field of view 264x384 mm2, 40-60 slices, readout bandwidth 590 Hz/Px, refocusing flip-angle 90°, 30 dynamics, total acquisition time 15.0-18.4 minutes). To facilitate a 3D distortion correction the vendor-provided 2D distortion correction was disabled.
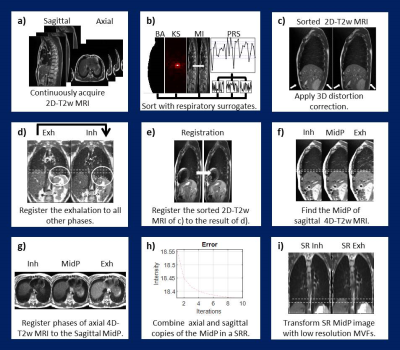
Figure 1 illustrates the workflow, as implemented in MATLAB (The MathWorks, MA). For both the axial and sagittal orientations an initial guess (i4D-T2w MRI) was obtained by sorting the data using image-based respiratory surrogates3-5 (body area, mutual information and artificial k-space) and applying a gradient 3D non-linearity correction. Stitching artefacts were reduced by 3D registration6 of the relatively unaffected exhalation phase to all other phases in the i4D-T2w MRI; yielding a stitching artefact-free 4D-T2w (s4D-T2w) MRI. Subsequently, i4D-T2w MRI was registered6 to the corresponding respiratory phase in the s4D-T2w MRI. The phases of the resulting corrected 4D-T2w MRI were transformed to MidP7 and combined using SRR8. Super-resolution 4D-T2w MRI was obtained by transforming the results of SRR with the motion information of the corrected sagittal 4D-T2w MRI.
To assess whether motion information was lost in the process, colour intensity projection images9 (CIPs), were calculated using all acquired dynamics and compared to those from the generated super-resolution 4D-T2w MRI.
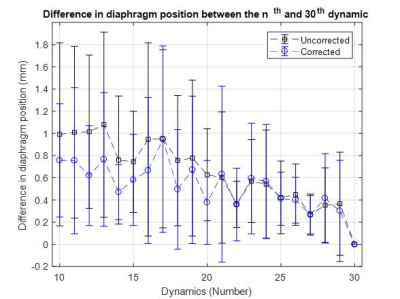
To find the required number of dynamic acquisitions needed for representative 4D-T2w MRI, MidP images were reconstructed (Figure 1.b-f) using a subset of the data ranging from 10 to 30 dynamics. For each MidP image, the diaphragm position was calculated using an edge-detection method10 and averaged over five slices. To separate the change in MidP appearance originating from a smaller number of dynamics from changes due to variation in the respiratory pattern, the drift in MidP was calculated from ground-truth data using the same edge-detection method and number of dynamics.
Results
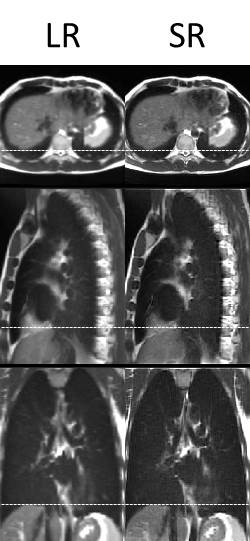
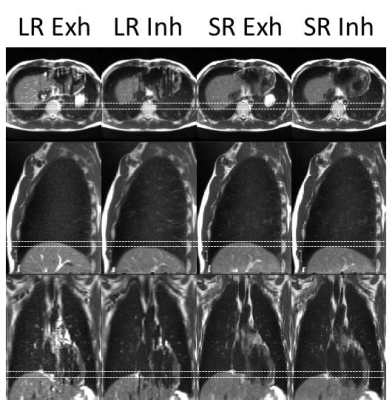
Super-resolution (1.0x1.0x1.0 mm3) distortion corrected 4D-T2w MRI was reconstructed in an automated workflow within 25 minutes. Generated 4D-T2w MRI did not suffer from data incompleteness and displayed reduced stitching artefacts. Figure 2 shows a comparison between super-resolution MidP-T2w MRI and the average interpolated sagittal MidP image. The in-plane quality of both the axial and sagittal orientations was well preserved in SRR; a reduction of blurring was observed in the coronal plane. The motion information exhibited by the corrected sagittal 4D-T2w MRI was well transferred to the results of SRR, as demonstrated in Figure 3.
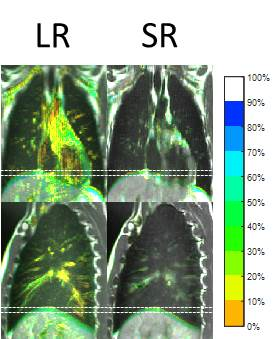
Figure 4 shows example CIPs. The diaphragmatic motion-range exhibited by the ground-truth CIPs was in good agreement with the motion-range resulting from SRR. Additionally, cardiovascular flow was averaged out in the SRR CIPs. Figure 5 displays the absolute mean (averaged over all subjects) differences in diaphragm position of the MidP reconstructed from a subset of the data, compared to the position using all 30 dynamics. The errors in position introduced by using fewer dynamic acquisitions were within 0.9 mm.
Discussion
The presented method enables a reduction in slice-thickness through SRR, mitigates stitching artefacts by smooth registrations and also circumvents the problem of data incompleteness. Cardiovascular motion is substantially reduced by the SRR, which leads to a reduction of obscuring features, thus facilitating delineation of tumours and organs at risk. Super-resolution MidP/4D-T2w MRI could be co-registered with MidP/4D-CT to support contouring of tumours which exhibit poor soft-tissue contrast in CT10 or could be utilised in MRIgRT workflows for treatment delivery1. The proposed method could also be applied to other fast 2D sequences such as balanced gradient echo techniques.
A high agreement within 0.9 mm in the corrected mean absolute diaphragm positions of the MidP obtained with 10-15 dynamic acquisitions, compared to all 30 dynamic acquisitions, was observed. These results suggest that on average 10-15 dynamics are sufficient to obtain representative high quality MidP MRI and motion data, which requires 5.0-7.5 minutes of acquisition time.
Conclusion
A motion-modelling and SRR method was devised and optimised to obtain high quality 4D/MidP-T2w MRI. Super-resolution 4D/MidP-T2w MRI could be employed to facilitate treatment delivery on hybrid MRIgRT systems.Acknowledgements
We thank Siemens Healthineers for providing us with the spherical harmonic coefficients of the MAGNETOM Aera. We also thank Nina Tunariu, Dow-Mu Koh and Matthew Orton for their help with data acquisition and image analysis. We acknowledge NHS funding to the NIHR Biomedical Research Centre and the Clinical Research Facility at The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust and the CR UK Cancer Imaging Centre grant C1060/A16464. We acknowledge funding from CR UK programme grant: C33589/A19727 and project grants: C347/A18365, C309/A20926. Martin Leach is an NIHR emeritus senior investigator.References
1. Kontaxis C, Bol G,
Stemkens B, et al. Towards
fast online intrafraction replanning for free-breathing stereotactic body
radiation therapy with the MR-linac. Phys Med Biol [Epub]. 2017.
2. Liu Y, Yin F-F, Chen N-k, Chu M-L, Cai J. Four dimensional magnetic resonance imaging with retrospective k-space reordering: A feasibility study. Med Phys. 2015;42:534-41.
3. Hu Y, Caruthers SD, Low DA, Parikh PJ, Mutic S. Respiratory amplitude guided 4-dimensional magnetic resonance imaging. Int J Radiat Oncol. 2013;86:198-204.
4. Paganelli C, Summers P, Bellomi M, Baroni G, Riboldi M. Liver 4DMRI: A retrospective image‐based sorting method. Med Phys. 2015;42:4814-21.
5. Hui C, Wen Z, Stemkens B, et al. 4D MR imaging using robust internal respiratory signal. Phys Med Biol. 2016;61:3472-87.
6. Vercauteren T, Pennec X, Perchant A, Ayache N. Diffeomorphic demons: Efficient non-parametric image registration. NeuroImage. 2009;45:S61-S72.
7. Wolthaus J, Sonke JJ, Van Herk M, Damen E. Reconstruction of a time‐averaged midposition CT scan for radiotherapy planning of lung cancer patients using deformable registration. Med Phys. 2008;35:3998-4011.
8. Irani M, Peleg S. Improving resolution by image registration. Graph Models Image Process. 1991;53:231-9.
9. Freedman JN, Collins DJ, Bainbridge H, et al. Evaluation of 4D-T2w MRI methods for lung radiotherapy treatment planning with application to an MR-linac. ISMRM 25th Annual Meeting & Exhibition. 2017;2906.
10. Freedman JN, Collins DJ, Bainbridge H, et al. T2-Weighted 4D Magnetic Resonance Imaging for Application in Magnetic Resonance-Guided Radiotherapy Treatment Planning. Invest Radiol [Epub]. 2017.
Figures