0112
Brown Adipose Tissue Mass Measurement by Z-Spectrum Imaging1Radiology, University of Illinois, Chicago, IL, United States, 2Bioengineering, University of Illinois at Chicago, Chicago, IL, United States, 3Center for MR Research, University of Illinois at Chicago, Chicago, IL, United States, 4Research Resource Center, University of Illinois at Chicago, Chicago, IL, United States, 5Radiology, Northwestern University, Chicago, IL, United States, 6Physiology and Biophysics, University of Illinois, Chicago, IL, United States
Synopsis
Brown adipose tissue (BAT) has a great relevance in metabolic diseases and has been shown to be reduced in obesity and insulin resistance patients. Currently, Dixon MRI is used to calculate fat-water fraction (FWF) and differentiate BAT from the less hydrated and more lipid-rich white fat. However, it may introduce fat-water swapping artifacts. Here, we showed that Z-Spectrum MRI can effectively measure FWF and BAT mass in vivo free from artifacts, due to the direct saturation of both water and fat protons.
Introduction
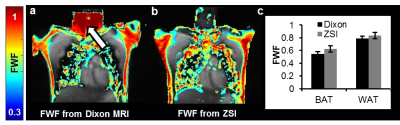
Brown adipose tissue (BAT) is a subtype of fat tissue different from the conventional white tissue (WAT). The task of BAT is in fact not the storage of fat as energy reservoir but energy production at the expense of fat1. Given its ability to burn fatty acids and glucose to generate heat, it has a great relevance in metabolic diseases and has been shown to be reduced in obesity and insulin resistance patients2. Measurement of BAT mass is key to study the disrupted metabolic homeostasis and potential treatment strategies. Currently, Dixon MRI is used to calculate fat-water fraction (FWF) and differentiate BAT, which has multilocular small lipid droplets immersed in watery environment, from the more lipid-rich and less hydrated WAT3. However, it may fail in areas due to phase wrapping and introduce fat-water swapping artifacts. Here, we aim to investigate the capacity of the Z-Spectrum MRI for the FWF measurement and the identification of BAT in vivo.Methods
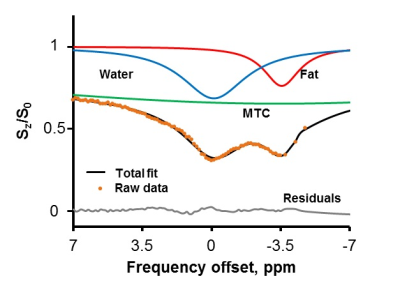
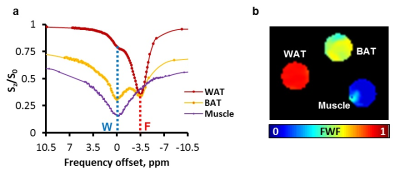
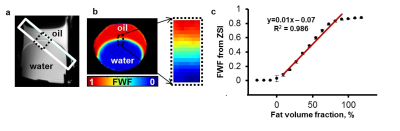
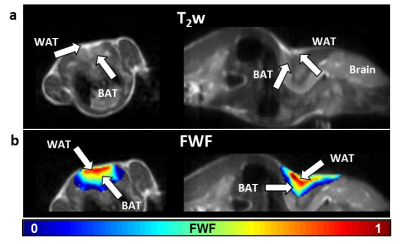
Z-Spectrum MRI was performed on in vitro WAT, BAT and lean tissues extracted from healthy mice. A chemical exchange saturation transfer (CEST) sequence was used to acquire Z-Spectra with a square saturation pulse of 3.5 μT for 1s and frequency offsets ranging from -5 to 5 ppm at intervals of 0.25 ppm, together with ±10, ±20, ±100 ppm offsets. The saturation pulse was followed by single-slice fast spin echo (FSE) readout. Z-Spectra data were fitted to a model with three Lorentzian peaks reflecting the direct saturation of tissue water (W, centered at 0 ppm) and methylene fat (F, centered at around −3.5 ppm from water), and the broad-band magnetization transfer from the semi-solid tissues. The peak amplitudes of water (W) and fat (F) were used to map the FWF, defined as F/(F+W). The novel FWF metric was calibrated with a oil and water mixture phantom and validated in mice and human subjects using the same imaging parameters. For the human study (n = 5), a Dixon MRI sequence with 6 echoes and IDEAL reconstruction was acquired in the same session to quantify the fat fraction distribution. FWF in known BAT and WAT regions derived from the two techniques were compared by Student’s t-tests.Results
Z-Spectra revealed two distinctive peaks corresponding to W and F. The novel FWF metric from Z-spectral fitting clearly differentiated in vitro WAT, BAT, and lean tissues which have FWF of roughly 1, 0.5, and 0 respectively. Calibration with oil mixture phantoms revealed a linear relationship between FWF and the actual fat fraction (R2=0.98). In vivo experiments in mice confirmed in vitro results by showing FWF around 0.6 in the known interscapular BAT and differentiated it from subcutaneous WAT (p<0.004). FWF maps of human subjects at 3T showed the same FWF distribution as Dixon MRI (p>0.07) except for occasionally observed fat-water swapping artifacts in Dixon’s FWF maps. ZSI is independent from B0 field inhomogeneity and free from fat-water swapping artifacts since both lipid and water frequency offsets are determined simultaneously during Z-Spectral fitting.Conclusion
We demonstrated that ZSI can derive artifact-free FWF maps, which can be used to identify BAT mass distribution in vivo non-invasively.Acknowledgements
We are grateful for the research supports provided by the Department of Radiology and the Center for MR Research at the University of Illinois at Chicago.
This research was supported by NIH under grant # R21 EB023516.
References
1. Herrero L. Fatty acid metabolism and the basis of brown adipose tissue function. Adipocyte. 2016;5(2):98–118.
2. Cypess AM, Haft CR, Laughlin MR, Hu HH. Brown Fat in Humans: Consensus Points and Experimental Guidelines. Cell Metab. 2015;20(3):408–15.
3. Gifford A, Towse TF, Walker RC, Avison MJ, Welch EB, Gifford A, et al. Characterizing active and inactive brown adipose tissue in adult humans using PET-CT and MR imaging. Am J Physiol Endocrinol Metab. 2016;i(2):95–104.
Figures