0111
Amide proton transfer-weighted imaging in meningioma: Prediction of tumor grade, histologic subtype and association with Ki-67 proliferation status1Department of Radiology, Zhujiang Hospital of Southern Medical University, Guangzhou, China, 2Division of MR Research, Department of Radiology, Johns Hopkins University School of Medicine, Baltimore, MD, United States
Synopsis
A correct preoperatively predicting grade histologic subtype and tumor proliferation of meningioma is important in clinic treatment. APT imaging are designed to assess brain tumor on the level of cell and molecule. In this study we hoping to explore if this technique was useful in evaluating the meningioma comprehensively.
Target audience
Researchers and clinicians who are interested in meningioma and functional MR imagingPurpose
Meningioma constitute approximately 36.7% of all primary central nervous system neoplasms[1]. Its therapeutic method and prognosis was related to tumor grade, histologic subtype and proliferation[2-5]. Conventional enhanced MRI can provide sufficient information to diagnose a meningioma. But the linear GBCA gadopentetate dimeglumine prone to deposit in the dentate nucleus and the globus pallidus, and thus may cause healthy risk[6; 7]. APTw MRI is novel functional modalities without GBCA. APT imaging is used to detect the amide protons of endogenous, low-concentration mobile proteins and peptides in tissue[8]. There was fewer research on using this technique in grading meningioma, but no research explores the use in predicting histologic subtype and tumor proliferation of meningioma. We aimed to explore if this technique was useful in comprehensive evaluation the meningioma.Methods
Local Ethics Committee approved the prospective study, and informed consent was obtained from all patients. Seventy-nine patients who underwent preoperative routine MRI scan as well as APTw MRI were retrospectively assessed. All MR scans were performed on a clinical MRI system (Achieva 3.0 T; Philips Medical Systems, Best, Netherlands). APT imaging was obtained using a fat-suppressed, turbo-spin-echo pulse sequence, with a pulse-train radiofrequency saturation (duration=200ms×4; inter-pulse delay, 10ms; power 2μT). Four Amide proton transfer-weighted (APTw) parameters were applied to calculate the solid component at the maximal slice of the tumor, the maximum APTw value (APTwmax), minimum APTw value (APTwmin), the maximum APTw value-minimum APTw value (APTwmax-min) and mean APTw value (APTwmean) were recorded. All cases were confirmed by postsurgical immunohistochemistry. The WHO grade I meningioma included nineteen fibroblastic, twenty meningothelial, nine transitional, and five microcystic meningiomas. The WHO grade II meningioma included twenty-three atypical, two clear-cell and one chordoid meningiomas. Independent-Samples T test followed by the Levene test was used to compare differences of 4 parameters between WHO grade I and WHO grade II meningioma group. The diagnostic performance of the 4 parameters was assessed with receiver operating characteristic (ROC) curve and area under the curve (AUC). A one-way analysis of variance (ANOVA) test followed by multiple comparisons of the Tukey test was used to compare differences of 4 parameters among 4 different subtypes of WHO grade I meningioma. Pearson’s correlation coefficient was used to analyze the association between APTwmax and Ki-67 index. P<0.05 was considered to be a statistically significant difference for all testsResults and discussion
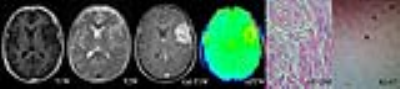
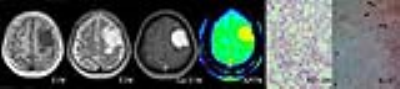
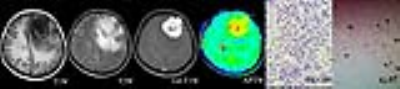
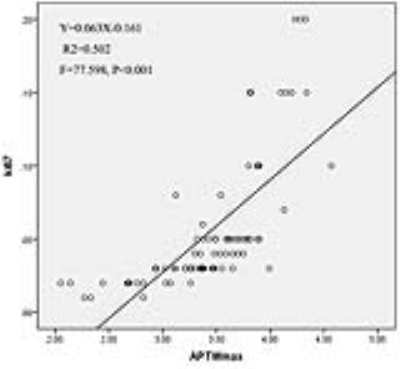
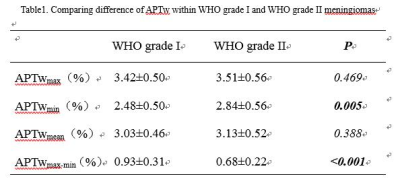
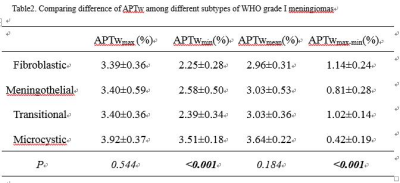
The APTwmin value for grade II meningiomas was higher (2.84%±0.56% vs. 2.48%±0.50%, P=0.005) and the APTwmax-min value was lower (0.68%±0.22% vs. 0.93%±0.31%, P<0.001) than the same parameters for grade I tumors (Fig.1,2,3 and Table 1). By using APTwmax-min as a discriminative index, the sensitivity and specificity were 64.2% and 88.5%, the accuracy was 72.2%, respectively. The APTwmin values and APTwmax-min values among different meningioma histologic subtypes were significantly different (F=6.825, P<0.001 and F=12.113, P<0.001, respectively) (Table 2). The APTwmax value was positively correlated with Ki-67 (r=0.705, P<0.001) in all meningiomas, and the regression equation for the Ki-67 index (Y) and APTwmax (X) was Y=0.063X-0.161 (F=77.598, P<0.001, R2=0.502) (Fig.4).Conclusion
APTw MRI is promising molecular imaging modalities based on pathophysiologic changing, which could assess meningioma on the level of cell and molecule, and provide more precise and microscopic of functional diagnostic information. We believe as a supplementary method in clinical settings would be valuable to improve the ability of evaluating meningioma more comprehensive.Acknowledgements
No acknowledgement found.References
1. Louis DN, Perry A, Reifenberger G, et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta neuropathologica 131:803-820
2. Lin Z, Zhao M, Ren X, et al (2017) Clinical Features, Radiologic Findings, and Surgical Outcomes of 65 Intracranial Psammomatous Meningiomas. World neurosurgery 100:395-406
3. Marciscano AE, Stemmer-Rachamimov AO, Niemierko A, et al (2016) Benign meningiomas (WHO Grade I) with atypical histological features: correlation of histopathological features with clinical outcomes. Journal of neurosurgery 124:106-114
4. Romani R, Tang WJ, Mao Y, et al (2014) Diffusion tensor magnetic resonance imaging for predicting the consistency of intracranial meningiomas. Acta Neurochir (Wien) 156:1837-1845
5. Santelli L, Ramondo G, Della Puppa A, et al (2010) Diffusion-weighted imaging does not predict histological grading in meningiomas. Acta Neurochir (Wien) 152:1315-1319; discussion 1319
6. Collidge TA, Thomson PC, Mark PB, et al (2007) Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 245:168-175
7. Ramalho J, Castillo M, AlObaidy M, et al (2015) High Signal Intensity in Globus Pallidus and Dentate Nucleus on Unenhanced T1-weighted MR Images: Evaluation of Two Linear Gadolinium-based Contrast Agents. Radiology 276:836-844
8. Zhou J, Lal B, Wilson DA, Laterra J, van Zijl PC (2003) Amide proton transfer (APT) contrast for imaging of brain tumors. Magn Reson Med 50:1120-1126
Figures