0110
Magnetic Resonance Temperature Imaging for Nanoparticle-Mediated Tumor Photothermal Therapy1Medical Imaging Department, First Affiliated Hospital of Xi'an Jiaotong University, Xi’an, China, 2Center for Molecular Imaging and Translational Medicine, Xiamen University, Xiamen, China, 3MR Research China, GE Healthcare, Beijing, China
Synopsis
Image-guided cancer therapy have the ability to integrate noninvasive imaging and minimally invasive interventions such as photothermal therapy (PTT) together to improve the precision of treatment. In the present study, we investigated magnetic resonance temperature imaging (MRTI) as a tool for non-invasive monitoring of tumor temperature distribution and changes during laser irradiation. To this end, we injected PEGylated graphene oxide (GO-PEG) to 4T1 tumor models and irradiated the tumors to induce PTT. Our studies demonstrate that MRTI, which is a feasible tool for the determination of temperature distribution, can be used to guide nanoparticle-mediated tumor photothermal therapy.
Introduction
Nanoparticle-mediated photothermal therapy (PTT), which has minimal invasiveness and high selectivity for inoperable tumors, has emerged as an important treatment modality1,2. A successful PTT should both include targeted delivery of nanomaterials to the tumor for effective thermal ablation under photoradiation, and noninvasive real-time monitoring of heat distribution to prevent damage to surrounding normal tissues3. Previous research on PTT has focused on its therapy efficiency and selectivity4,5. Noninvasive real-time temperature monitoring, which is pivotal for the assessment of the extent of tissue damage and the enhancement of the safety of treatment, however, have been rarely studied.
MR temperature imaging (MRTI) allows to real-time assessment of thermal-based therapies6. In addition to excellent soft tissue contrast for tumor delination, MRTI is also capable of monitoring deep tissue energy transfer and therefore becomes a preferred method for thermal treatment temperature monitoring7,8. The water proton resonance frequency shift (PRF) approach is often used for MR temperature mapping because of its high spatial resolution and short acquisition time9. In the PRF method, temperature dependence comes from weak local magnetic field shielding caused by the moving electron clouds around the proton. With temperature increase, the screening effect of bounded electrons of the proton is increased, which weakens the local magnetic field of the proton further and shows the negative shift of water PRF. In this study, we used PRF method to investigate GO-PEG mediated PTT for the purposes of analysis the resulting spatiotemporal temperature distributions during laser irradiation in mouse 4T1 breast tumor xenografts associated histology.
Materials and Methods
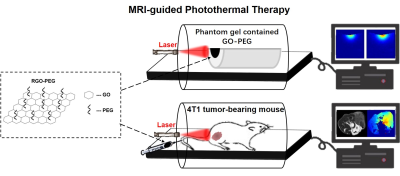
We investigated the feasibility of PTT for 4T1 tumor-bearing mice under the guidance of MR temperature imaging using GO-PEG. A 3.0 T clinical MR scanner (GE Discovery 750w, Milwaukee, WI) equipped with a 8 channels rat coil of 50 mm inner diameter (Shanghai Chenguang Medical Technologies Co. Ltd,Shanghai) was used to monitor laser-induced thermal gradient temperature distribution of tumor tissue. Using the PRF method, thermal maps were validated for in vivo thermal measurement with GO-PEG. All the experiments were divided into two parts: in vitro system evaluation through temperature measurement of phantom gel and in vivo PTT (Figure 1).
During MRI scanning, all the mice were anesthetized by intraperitoneal injection of 2% Nembutal Sodium (50mg/Kg) and placed with an integrated heating system. MR images were acquired using the following parameters: (1) T2-weighted images using a fast spin echo sequence, TR/TE=2300/120ms, FOV=50×50mm, Matrix=160×160, Average=4, Slice thickness=2.0mm, Echo train length 18; (2) MRTI using a SPGR sequence, TR/TE=100/3.8ms, FA=30°, FOV=50×50mm, Matrix=128×128, Average=1, Slice thickness=2.0 mm. All data processing was performed by using in-house developed script in MATLAB (Mathworks, Nattick, MA). For better assessment of the extent of tumor necrosis, mice was sacrificed 24 hrs post-PTT and tumor was removed for histological analysis.
Results
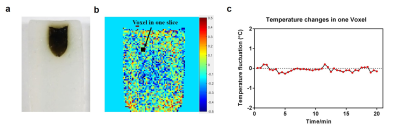
The thermal image maps calculated by Matlab were represented using a color-coded lookup table scheme. Figure 2a shows the real view of phantom gel contained GO-PEG. For the system stability evaluation, the temperature change was measured under the constant conditions. The acquired 40 phase images during laser irradiation were used to measure MRI field drift as observed in the signal phase changes (Figure 2b). According to our measurement, the maximum temperature change was less than 0.3°C (Figure 2c).
In order to observe the temperature change profile, the gel phantom was irradiated at a laser power density of 1 W/cm2 for 10 min with the fiber oriented parallel to the main magnetic field B0. The MRI signal was recorded during the process. To further confirm the accuracy of MRTI, the phantom was taken out from the MRI chamber and irradiated with the same method. Thermal images were obtained real-time by an FLIR A35-series camera (FLIR Systems Inc., Wilsonville, OR) and quantified by BM_IR software. Figures 3a and 3b show MRTI has high accuracy compare to near-infrared temperature imaging.
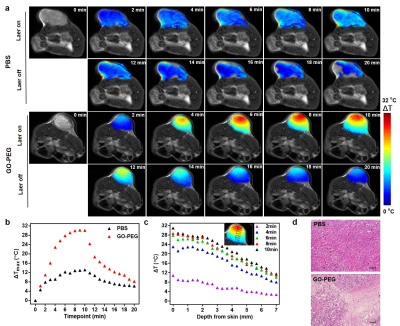
Calculated thermal maps are overlaid onto T2 images of the mouse xenografts (Figure 4a). Relatively low heating is observed in the phosphate buffer saline (PBS) control versus the highly tumor specific heating observed in the GO-PEG tumor. The GO-PEG tumors also heated faster and maintained increased temperatures deeper into the tumor than control subjects (Figure 4b–c). And figure 4d shows the temperature distribution is highly consistent with the extent of tumor thermal necrosis.
Conclusion and Discussions
This research has demonstrated that the PRF-based MRTI method can be used to guide GO-PEG mediated laser ablation in 4T1 xenografts mice. MRTI provides an effective way of monitoring the intratumoral spatiotemporal distribution of temperature in real time. Future studies will be focused on larger tumor models and interstitial delivery. In conclusion, MRTI-guided PTT is found to be a promising method for future PTT evaluation and clinical translation.
Acknowledgements
This study was supported by the National Key Research and Development Program of China (2016YFC0100300), National Natural Science Foundation of China (No. 81471631, 81771810 and 51706178), the 2011 New Century Excellent Talent Support Plan of the Ministry of Education, China (NCET-11-0438) and the Clinical Research Award of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF-CRF-2015-004).References
1. O'Neal, D. P.; Hirsch, L. R.; Halas, N. J. , et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer letters 2004, 209 (2), 171-6.
2. Lal, S.; Clare, S. E.; Halas, N. J., Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Accounts of chemical research 2008, 41 (12), 1842-51.
3. Zhou, M.; Melancon, M.; Stafford, R. J., et al. Precision Nanomedicine Using Dual PET and MR Temperature Imaging-Guided Photothermal Therapy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine 2016, 57 (11), 1778-1783.
4. Melancon, M. P.; Zhou, M.; Li, C., Cancer theranostics with near-infrared light-activatable multimodal nanoparticles. Accounts of chemical research 2011, 44 (10), 947-56.
5. Fu, G.; Zhu, L.; Yang, K., et al. Diffusion-Weighted Magnetic Resonance Imaging for Therapy Response Monitoring and Early Treatment Prediction of Photothermal Therapy. ACS applied materials & interfaces 2016, 8 (8), 5137-47
6. Quesson, B.; de Zwart, J. A.; Moonen, C. T., Magnetic resonance temperature imaging for guidance of thermotherapy. Journal of magnetic resonance imaging : JMRI 2000, 12 (4), 525-33.
7. Carpentier, A.; McNichols, R. J.; Stafford, R. J., et al.Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 2008, 63 (1 Suppl 1), ONS21-8; discussion ONS28-9.
8. Raz, O.; Haider, M. A.; Davidson, S. R., et al. Real-time magnetic resonance imaging-guided focal laser therapy in patients with low-risk prostate cancer. European urology 2010, 58 (1), 173-7.
9. McDannold, N., Quantitative MRI-based temperature mapping based on the proton resonant frequency shift: review of validation studies. International journal of hyperthermia : the official journal of European Society for Hyperthermic Oncology, North American Hyperthermia Group 2005, 21 (6), 533-46.
Figures