0109
Hollow Manganese-Silicate (HMS) Nanoparticles as a Liver Specific MRI contrast agent1Department of Radiology and Center for Imaging Science, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea, 2National Creative Research Initiative Center for Nanospace-confined Chemical Reactions and Department of Chemistry, Pohang University of Science and Technology (POSTECH), Gyeongbuk, Republic of Korea, 3Departments of Health Science and Technology and Medical Device Management and Research, Samsung Advanced Institute for Health Science and Technology, Sungkyunkwan University, Seoul, Republic of Korea
Synopsis
In this study, we demonstrate hollow manganese silicate nanoparticle (HMS) as a liver specific magnetic resonance imaging (MRI) contrast agent. HMS releases Mn2+ ions in the acidic physiological condition, which can be utilized for characterizing different tumor types.
Purpose & Introduction
We demonstrate hollow manganese silicate nanoparticle (HMS) as a liver specific magnetic resonance imaging (MRI) contrast agent. HMS releases Mn2+ ions in the acidic physiological condition, which can be utilized for characterizing different tumor types. HMS would be readily taken up by the kupffer cells of the liver due to the nanoparticulate nature1,2 and exert the burst-release of T1-contrast enhancing Mn2+-ions from the cell-internalized HMS.3 Based on these characteristics, HMS can induce time-sequential T1-weighted MR imaging which might be influenced by the nature of various tissues in the liver. In this study, we demonstrate the effectiveness of the burst-releasing Mn2+ ions of HMS as a liver-specific MR contrast agent in characterizing hepatic tumors, which was evaluated through the in-depth MR imaging study with various hepatic tumor models.Materials and Methods
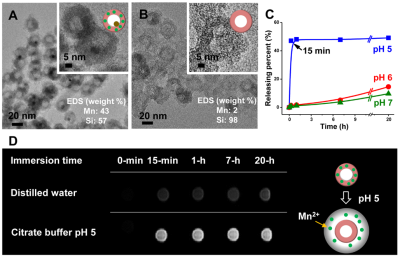
Synthesis of HMS: The HMS with the yolk-shell structure, containing an encapsulated Cu nanocrystal (9 ± 1 nm) inside cavity (14 ± 2 nm) of the hollow manganese-silicate shell (39 ± 2 nm), was synthesized as previously reported, by preparing MnO@SiO2/Cu2+, annealing it, and selectively etching the outer silica shell of the hollow-structure NPs (Cu@HSNPs, Figure 1A).4 The surface of the HMS was then coated with PEG (Mw ~ 600). When the PEG-modified HMS, containing as much as 19.3 wt% of Mn was immersed in a buffer suspension at pH 5.0, all dischargeable Mn2+ ions were immediately released and rapidly increased the concentration of free Mn2+-ions to a maximum within 15-min (Figure 1B). In contrast, the Mn2+-release was very slow in either buffer solution at pH 6.0 or distilled water (Figure 1C). Figure 1D show the T1WIs of HMS immersed in distilled water and the buffer solution at pH 5.0 caused immediate increase in the signal intensity. This change signifies the triggering of MR contrast enhancement by Mn2+-outpouring from HMS in acidic solution (Figure 1D).
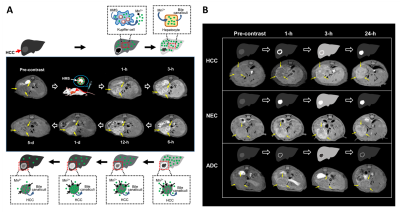
Animal Models: To assess the possibility of distinguishing various hepatic tumors by using HMS as a contrast agent, we employed three malignant hepatic tumor models, including HCC as hepatocellular tumor (HepG2 cells), and NEC (neuroendocrine carcinoma, STC-1 cells) and ADC (colon adenocarcinoma, HT29 cells) as hypervascular and hypovascular non-hepatocellular metastases, respectively. Eash tumor cells mixed with Matrigel in a total volume of 10 μl were slowly inoculated into the mouse liver. T2-wighted MRIs of these hepatic tumors were performed to confirm the presence of the tumors after 4 weeks of growth.
MRI Acquisitions: Pre-contrast T1-weighted images (T1WIs) were obtained, and then HMS (at 3 mg Mn/kg of body weight) was delivered by intravenous injection via the tail vein. All in vivo MR imaging was performed on a 7T/20 MR System (Bruker-Biospin, Fallanden, Switzerland) equipped with a 20-cm gradient set capable of supplying up to 400 mT/m in a 100‑µs rise time. A quadrature volume coil (35 mm i.d.) was used for excitation and to receive the signal. After post-injection, time-sequential T1WIs of each mouse liver were obtained using a fast spin echo T1-weighted MR sequence (TR/TE = 380/7.7 ms, NEX = 8, echo train length = 2, in-plane resolution 100×100 mm with a slice thickness of 1 mm and 14 slices) with respiratory gating.
Results and Discussion
In all animal models, time-sequential enhancement changes of the liver parenchyma on the T1WIs become hyperintense from 1-h to 6-h after the HMS injection, with the peak point at 3-h post-injection (Figure 2). Contrary to that of the liver parenchyma, T1WIs of the tumor tissues (e.g., HCC, NEC and ADC tissues) after the HMS injection were apparently distinct one another, demonstrating their own characteristic contrast enhancement patterns (Figure 2B) depending the tumor types. HCC tissues exhibited contrast enhancement only at the periphery during the initial 1-h and then demonstrated the centripetal and progressive fill-in enhancement pattern of heterogeneously hyperintense mass over following 24-h, while remaining necrotic portions remained unenhanced (Figure 2). On the other hand, NEC tissues showed the strong and persistent enhancement pattern of heterogeneously hyperintense mass that contained some hypointense necrotic portions, whereas ADC tissues showed a poor enhancement pattern of homogeneously hypointense mass with a thin rim-enhancement during the entire period (Figure 2B).Conclusion
This study demonstrates the unique capability of the newly developed HMS as a liver specific MRI contrast agent. HMS induces burst-release of Mn2+ ions from Kupffer cells, which then enter to the tumor tissues to activate time-sequential enhancement changes on the T1WIs. HMS generates the tumor type dependent enhancement patterns of different hepatic tumors, which may be attributed to tumor vascularity, cell density, hepatocellular specificity, mitochondria activity, and etc. Overall, HMS suggests new kind of MRI contrast agent that is based on Mn2+ ions when there is an increasing concern about latent toxicities of Gd3+ based contrast agents.Acknowledgements
This work was supported by Samsung Research Funding Center of Samsung Electronics under Project Number SRFC-MA1402-05.References
1. Feliu N, Docter D, Heine M, et al. In vivo degeneration and the fate of inorganic nanoparticles. Chem. Soc. Rev. 2016;45(9):2440-2457.
2. Huang J, Bu L, Xie J, et al. Effects of nanoparticle size on cellular uptake and liver MRI with polyvinylpyrrolidone-coated iron oxide nanoparticles. ACS Nano 2010;4(12):7151-7160.
3. Kim SM, Im GH, Lee DG, et al. Mn(2+)-doped silica nanoparticles for hepatocyte-targeted detection of liver cancer in T1-weighted MRI. Biomaterials 2013;34(35):8941-8948.
4. Kim JG, Kim SM, Lee IS. Mechanistic Insight into the Yolk@Shell Transformation of MnO@Silica Nanospheres Incorporating Ni(2+) Ions toward a Colloidal Hollow Nanoreactor. Small 2015;11(16):1930-1938.
Figures