0108
Probing perturbed hepatic metabolism in bile-duct-ligated rats with hyperpolarized 13C pyruvate and arginine1Laboratory for Functional and Metabolic Imaging, Swiss Federal Institute of Technology, Lausanne (EPFL), Lausanne, Switzerland, 2Department of Pharmacology and Toxicology, University of Lausanne, Lausanne, Switzerland, 3Centre d'Imagerie Biomedicale (CIBM), Swiss Federal Institute of Technology, Lausanne (EPFL), Lausanne, Switzerland, 4Laboratory for Functional and Metabolic Imaging & Centre d'Imagerie Biomedicale (CIBM), Swiss Federal Institute of Technology, Lausanne (EPFL), Lausanne, Switzerland
Synopsis
Detoxification of ammonia by the urea cycle and maintenance of glucose homeostasis by gluconeogenesis are two critical functions of the liver. The bile duct ligation (BDL) model of cirrhosis was used to test the ability of hyperpolarized [6-13C]arginine and [1-13C]pyruvate to detect changes in liver function. The conversion of hyperpolarized L-[6-13C]arginine to 13C-urea was observed in a sham-operated rat but not in BDL rats. Striking differences in pyruvate metabolism between the two groups were also noted, indicating that these probes can sense changes in hepatic mitochondrial and cytoplasmic metabolism associated with biliary cirrhosis.
Introduction
Detoxification of ammonia by the urea cycle and maintenance of glucose homeostasis by gluconeogenesis are two critical functions of the liver. Hyperpolarized [1-13C]pyruvate is converted in the liver to 1- and 4-carbon intermediates of gluconeogenesis,1,2 and hyperpolarized L-[6-13C]arginine has been used to probe arginase,3 which catalyzes the final step of the urea cycle. The bile duct ligation (BDL) model of biliary cirrhosis shows progressively deteriorating liver function, affecting both urea synthesis4 and gluconeogenesis,5 and it was used here in a pilot study to test the ability of hyperpolarized [6-13C]arginine and [1-13C]pyruvate to detect these metabolic changes in the rat liver.Methods
Animals: BDL and sham-operated rats were prepared as described6 and monitored over 6 weeks, following blood glucose, bilirubin, and GOT and GPT enzyme levels. Rats were anaesthetized with isoflurane (4%, then 1-2%) and catheters were installed for intravenous infusion and invasive blood pressure monitoring. After placing the rat on its back on the MRI scanner holder, a respiration pillow and rectal thermometer were installed and a surface coil (single-loop 1H, quadrature 13C) placed over the liver. Body temperature was maintained with circulating warm water.
Polarization: A sample cup was loaded separately with [1-13C]pyruvic acid doped with 21 mM OX063 radical, L-[6-13C]arginine (HCl, 2.8 M, 25 mM OX063), and 10 N NaOH to neutralize, and then polarized at 7 T, 1 K with 196.59 GHz microwave irradiation (50 mW). The sample was rapidly dissolved in superheated D2O phosphate saline buffer, automatically transferred to a phase separator/infusion pump in the scanner bore and 1.2 ml was infused into the rat intravenously over ~9 s.
MR acquistion: Rats were scanned in a 9.4T horizontal bore magnet (Magnex), with a VNMRS console (Varian). Shimming was performed using FAST(EST)MAP and coil placement was confirmed by GEMS 1H imaging. Acquisition of the coil-localized hyperpolarized 13C metabolic NMR data was triggered by the start of the hyperpolarized pyruvate / arginine infusion. A series of respiration-gated pulse-acquire spectra (TR ≈ 3 s, BIR4 30° adiabatic pulse) were acquired.
Data analysis: Metabolite spectral peaks in individual spectra were fit using Bayes and time courses plotted to compute the areas-under-the-curve (AUCs) for each metabolite, which were normalized either to the substrate signal (arginine in case of urea) or the total pyruvate and pyruvate metabolite signal.
Results and Discussion
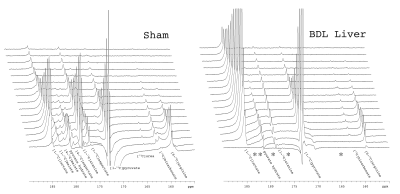
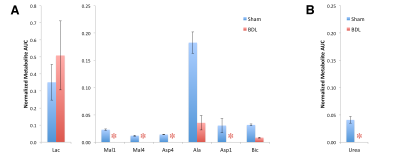
At six weeks post surgery the BDL rats showed enlarged livers and signs of compromised function, including elevated bilirubin, liver enzymes and lower blood glucose. A 13C-urea peak (165.47 ppm) was observed scanning the liver of a sham-operated animal after the infusion of hyperpolarized [6-13C]arginine (159.72 ppm) co-polarized with [1-13C]pyruvate (Fig 1) (n = 2). Urea production was also detected when scanning the liver of healthy rats infused with [6-13C]arginine. By contrast, no urea was apparent in BDL-rats, even when all the spectra containing metabolite signals were summed. The expected hepatic metabolic products of [1-13C]pyruvate, including [1-13C]lactate, [1-13C]- and [4-13C]malate, [1-13C]alanine, [1-13C]- and [4-13C]aspartate, and 13C-bicarbonate were apparent when scanning the sham-operated rat. By contrast, the 4-carbon metabolites downstream of pyruvate carboxylase were completely absent in the BDL-rats, with conversion to lactate dominating, and small alanine and bicarbonate signals also detected (Fig 2). The lack of 4-carbon metabolites is consistent with the diminished gluconeogenic capacity of the cirrhotic liver.
The effect of biliary cirrhosis on hepatic arginase activity is not clear, although arginase transcript levels are reportedly unaffected.7 The lack of conversion of hyperpolarized arginine to urea observed here is likely due to decreased hepatic arginase activity, decreased arginine uptake, or a combination thereof.
Conclusions
This pilot study clearly demonstrates the ability of hyperpolarized 13C pyruvate and arginine to detect changes in carbohydrate, amino-acid and nitrogen metabolism in a rat model of biliary cirrhosis. Longitudinal studies will test their ability to detect changes in hepatic metabolism soon after bile-duct ligation.Acknowledgements
Stefanita Mitrea, Analina da Silva (CIBM) and Dario Sessa (HUG) for animal preparation. Dr. O. Braissant (CHUV) and Dr. V. McLin (HUG) our collaborators on the BDL project. Supported by CIBM of the UNIL, UNIGE, HUG, CHUV, EPFL, the Leenaards and Jeantet Foundations and SNSF project no 310030_173222/1.References
1. Moreno KX, Moore CL, Burgess SC, et al. Production of hyperpolarized (13)CO2 from [1-(13)C]pyruvate in perfused liver does reflect total anaplerosis but is not a reliable biomarker of glucose production. Metabolomics. 2015;11(5):1144–56.
2. Emine Can, Hikari A.I. Yoshihara, Jessica A.M. Bastiaansen, Ret al. Effects of 3-MPA on in vivo hepatic metabolism of hyperpolarized [1-13C] pyruvate . Abstract 0166 ISMRM 2017 Honolulu http://cds.ismrm.org/protected/17MPresentations/abstracts/0166.html
3. Najac C, Chaumeil MM, Kohanbash G, et al. Detection of inflammatory cell function using (13)C magnetic resonance spectroscopy of hyperpolarized [6-(13)C]-arginine. Sci Rep. 2016;6:31397.
4. Nielsen SS, Grøfte T, Tygstrup N, et al. Cirrhosis and endotoxin decrease urea synthesis in rats. Hepatol Res. 2007;37(7):540–7.
5. Krähenbühl S, Krähenbühl-Glauser S, Stucki J, et al. Stereological and functional analysis of liver mitochondria from rats with secondary biliary cirrhosis: impaired mitochondrial metabolism and increased mitochondrial content per hepatocyte. Hepatology. 1992;15(6):1167–72.
6. Biecker E, De Gottardi A, Neef M, et al. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313(3):952–61.
7. Nielsen SS, Grøfte T, Tygstrup N, et al. Cirrhosis and endotoxin decrease urea synthesis in rats. Hepatol Res. 2007;37(7):540–7.
Figures