0107
In-vivo metabolism of co-hyperpolarized [1-13C] pyruvate and [1,3-13C] acetoacetate identifies cytosolic and mitochondrial redox in ischemic perfused hearts1Advanced Imaging Research Center, University of Texas Southwestern Medical Center, Dallas, TX, United States, 2Department of Radiology, University of Texas Southwestern Medical Center, Dallas, TX, United States, 3Department of Internal Medicine, University of Texas Southwestern Medical Center, Dallas, TX, United States, 4Department of Chemistry, University of Texas at Dallas, Dallas, TX, United States
Synopsis
Cellular redox state is intricately linked with cardiac ischemia. Historically, tissue levels of lactate to pyruvate and β-hydroxybutyrate to acetoacetate have been used as indices of cytosolic and mitochondrial redox, respectively. The present study was designed to evaluate the potential of using co-hyperpolarized (HP) [1-13C] pyruvate and [1,3-13C] acetoacetate as reporters of cytosolic and mitochondrial redox, respectively. 13C NMR spectra of perfused rat hearts displayed increased production of both HP-lactate and HP-β-hydroxybutyrate in low flow ischemia, rotenone, and
Purpose
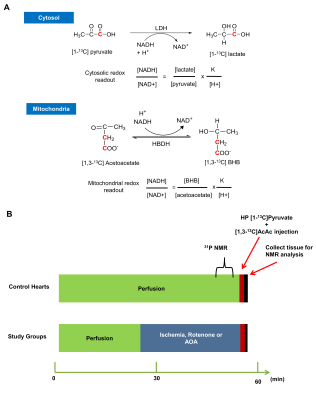
The cellular redox state plays a critical role in preventing cardiac myocardial damage1. Noninvasive tools for monitoring cellular redox are could aid in the diagnosis, treatment, and management of cardiac ischemia. Substrate metabolism can now be investigated in real-time using hyperpolarized (HP) 13C-labelled metabolic probes, providing new opportunities for analyzing cellular redox2. In this study, we used pyruvate «» lactate and acetoacetate «» β-hydroxybutyrate interconversions as a readout of cytosolic and mitochondrial redox, respectively (Fig. 1A).Methods
Heart perfusions and hyperpolarized 13C NMR: Animal studies were approved by the Institutional Animal Care and Use Committee (IACUC). Metabolism of HP-[1-13C]pyruvate and HP-[1,3-13C]acetoacetate was investigated in Sprague Dawley rats in four groups, control hearts (n=3), low-flow ischemic hearts (n=3), rotenone (mitochondrial complex-I inhibitor) treated hearts (n=2), and aminooxyacetate (AOA-inhibitor of transaminase activity) treated hearts (n=2). Hearts were excised and perfused (Fig.1B) using the Langendorff method and perfused with a modified Krebs-Henseleit (KH) solution (no Pi) containing a physiological mixture of glucose (5.5 mM), lactate (1.2 mM), pyruvate (0.12 mM), and long-chain free fatty acids (0.4 mM) with 0.75% bovine serum albumin, and oxygenated with a 95:5 mixture of O2/CO2 at a constant pressure of 100 cm-H2O 3. The perfusion pressure was lowered to 25 cm H2O for the low-flow ischemia hearts. Shimming was performed using the 23Na-FID to a linewidth of ~14 Hz while the heart was surrounded by a sucrose flush (250 mM) to remove extracellular 23Na. 31P-NMR spectra were acquired for intracellular pH measurements as described previously3. A solution (3 mL) containing [1-13C]pyruvate and [1,3-13C]acetoacetate (2mM each) was polarized in a HyperSense DNP instrument for 2 h using 15 mM OX063 and 2 mM ProHance. The sample dissolved quickly using 20 mL of Krebs-Henseleit buffer and injected directly into the perfusion media through a polyethylene catheter. A series of 13C NMR spectra were acquired immediately (2s delay time) after the injection of the hyperpolarized mixture using 20° pulses at 9.4 T (Varian INOVA) spectrometer equipped with a 25-mm broadband probe (Doty Scientific). After the 13C measurements, the hearts were freeze-clamped. The spectral data were processed using ACD Labs SpecManager and peak areas were normalized to the total peak areas of all 13C signals from the respective injected HP compound.
Quantitative 1H NMR analysis of tissue extracts: Frozen heart tissues were pulverized and extracted with 5% perchloric acid and then neutralized, freeze-dried, and reconstituted in D2O containing 0.5 mM DSS as an internal standard. NMR spectra were acquired on a 14.1 T spectrometer equipped with a 10-mm Bruker cryoprobe. The FID’s were zero-filled to 64k data points, Fourier-transformed, referenced to DSS, phased and baseline corrected using ACD/NMR Processor. The tissue concentrations were calculated as described3.
Results and Discussions
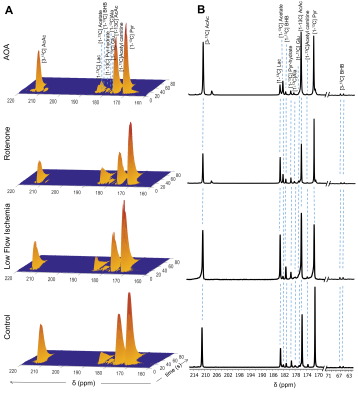
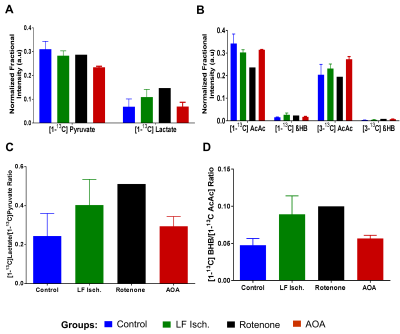
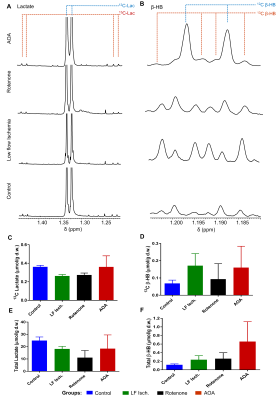
Oxygen consumption (µmol/min/g ww) measured in each heart ~5 min before injection of the HP substrates were: control (17.54±1.15), low-flow ischemia (7.63±2.26), rotenone-treated (5.67±0.55) and AOA treated (9.54±1.57). The extent of myocardial ischemia in the low flow ischemia, rotenone-treated, and AOA treated hearts was shown by a marked increase in [Pi]/[ATP] in 31P NMR spectra. Production of HP-[1-13C]lactate from HP-[1-13C]pyruvate and HP-[1,3-13C]β-hydroxybutyrate(β-HB) from HP-[1,3-13C]acetoacetate (AcAc) was monitored by 13C NMR spectroscopy (Fig. 2 and 3). Resonances characteristic of other metabolites were also observed. The ratios of both HP-[1-13C]lactate / HP-[1-13C]pyruvate and HP-[1-13C]β-HB / HP-[1-13C]AcAc ratios were higher in the low-flow ischemia, rotenone-treated and AOA treated hearts compared to control hearts (Fig. 3). However, the tissue concentration (Fig. 4) of lactate in low-flow ischemia, rotenone-treated and AOA treated hearts were no different. Interestingly, the tissue concentration of β-hydroxybutyrate was increased in low flow ischemia, rotenone-treated and AOA treated hearts in parallel with the HP observations. The changes in tissue levels of β-hydroxybutyrate in the treated heart groups suggest that mitochondrial redox is more sensitive to cardiac ischemic than is cytosolic redox.Conclusion
Increases in both cytosolic and mitochondrial redox were detected in low-flow ischemia, rotenone and AOA treated groups compared to control hearts by real-time 13C NMR. The tissue extract data suggest that mitochondrial redox is more sensitive to cardiac ischemia than is cytosolic redox. These results indicate that co-polarized pyruvate and acetoacetate can be used as metabolic markers of tissue redox in isolated perfused hearts.Acknowledgements
This work supported by the NIH (5R37-HL034557 and 8P41-EB015908). Xiaodong Wen and Thomas Hever are acknowledged for their technical support in the perfusion experiments.References
1. Burgoyne JR, Mongue-Din H, Eaton P, et al. Redox signaling in cardiac physiology and pathology. Circ Res. 2012;111(8):1091-1106.
2. Rider OJ, Tyler DJ. Clinical Implications of Cardiac Hyperpolarized Magnetic Resonance Imaging. Journal of Cardiovascular Magnetic Resonance. 2013;15(1):93.
3. Khemtong C, Carpenter NR, Lumata LL, et al.
Hyperpolarized 13C NMR detects rapid drug-induced changes in cardiac
metabolism. Magn Reson Med. 2015;74(2):312-319.
Figures