0105
In Vivo Molecular Imaging of MUC1-Expressing Colorectal Tumors Using Targeted Hyperpolarized Silicon Particles1The University of Texas MD Anderson Cancer Center, Houston, TX, United States, 2Rowan University, Glassboro, NJ, United States, 3Rice University, Houston, TX, United States
Synopsis
Silicon nano- and microparticles are well-suited for targeted molecular imaging, due to their biocompatibility, easily modifiable surface, and long-lasting 29Si MR signal (which can be enhanced by several orders of magnitude via dynamic nuclear polarization). We demonstrate targeted molecular imaging of human MUC1-expressing colon tumors in orthotopic mouse models using hyperpolarized 29Si MRI. The particles were able to selectively bind to MUC1-expressing tumors compared to controls, and the results were confirmed via histology of the excised tissue. The goal is to develop these targeted particles as a platform technology that will allow non-invasive screening of colorectal cancer using 29Si MRI.
Introduction
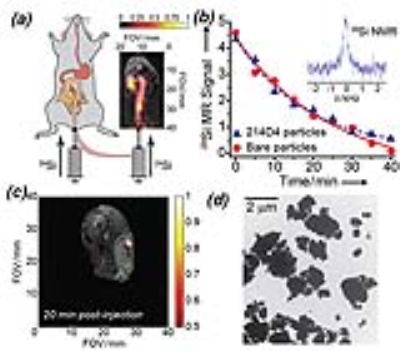
Colorectal cancer is the second-leading cause of cancer-related mortality in the United States [1], despite the prevalence of existing screening measures, such as the colonoscopy. In addition to the risk of intestinal perforation, traditional colonoscopies are also poorly suited to detect nonpolypoid colorectal neoplasms (i.e., ‘flat lesions’) [2]. Furthermore, the recent increase in colorectal cancer prevalence amongst traditionally younger populations (<50 yrs) [3] incentivizes the need for advanced screening methods that will allow early detection, observation of treatment efficacy, and monitoring of disease recurrence for colorectal cancer. Silicon nano- and micro-particles are potentially suitable molecular imaging agents, as they are non-toxic, easily functionalized for targeting, and can accommodate drug payloads [4,5]. Indeed, silicon particles are currently under clinical trial as drug delivery vehicles [6]. 29Si MRI can be used for in vivo tracking of silicon particles [7] that have been hyperpolarized via dynamic nuclear polarization (DNP), a method that temporarily increases the MRI signal by 4-5 orders of magnitude through enhanced nuclear spin alignment [8]. The resulting enhanced 29Si MR signal is preserved within the core of the particle [9], and is retained for significantly longer than other hyperpolarized contrast agents in vivo; typical T1 values range from 20-40 minutes [10]. We have previously demonstrated that silicon particles conjugated with tumor-targeting antibodies have equivalent hyperpolarization characteristics as bare particles, and HP 29Si MR signal could be detected in subcutaneous colorectal tumors for at least 20 minutes post-injection (Figure 1). Here we demonstrate that in vivo 29Si MRI can be used to detect silicon particle targeting in orthotopic Mucin-expressing colorectal tumors in mice.Methods
Silicon particles (2 µm) were surface functionalized with a 214D4 antibody that targets the large glycosylated ectodomain of human MUC1—a transmembrane Mucin protein that is overexpressed in some forms of colorectal cancer. The particles were hyperpolarized using a laboratory-constructed 29Si solid-state DNP device [10]; imaging studies were performed on a 7T small animal MRI. Following hyperpolarization, the targeted particles (in ~300 µL PBS) were rectally administered to transgenic mice that spontaneously produced human MUC1-expressing colorectal tumors in the lower intestinal tract, followed by a 10-15 minute wait prior to imaging. Co-registered [1H:29Si] MRI was performed using a dual-tuned 29Si/1H Litz coil: in vivo 29Si imaging used a coronal RARE sequence (α = 90°; TR/TE: 60 ms/1.8 ms; 6.4 cm FOV; 2 mm resolution), while 1H imaging utilized a coronal RARE scan (α = 90°), TR/TE: 1927 ms/9.5 ms with a RARE factor of 8; 6.4 cm FOV (0.25 mm resolution) and 4 averages. Experiments (n=3) were repeated with relevant chemical (non-targeting particles) and biological (non-MUC1-expressing tumors) controls. Following imaging, the mice were sacrificed and tissues were collected for histological analysis.Results
Silicon particles conjugated with the antibody were shown to target MUC1-expressing colorectal tumor cells in vitro both before and after hyperpolarization, demonstrating that the targeting ability of the antibody was not affected by the harsh conditions of DNP (Figure 2). The presence of tumors in mouse models was confirmed prior to MRI scans using a veterinary endoscope. Imaging studies of the targeted particles in MUC1-expressing mice demonstrate bright 29Si MR signal at the tumor sites, while the controls provided weaker, sporadic signal that was not associated with the presence of tumors (Figure 3). These results were correlated with tissue histology.Discussion
Demonstration that the targeting veracity of the antibody is not negatively affected by the harsh conditions of DNP is a critical step in developing hyperpolarized silicon particles as targeted imaging agents, not just for colorectal cancer but also as a platform technology for interrogating a variety of disease systems. The in vivo studies presented here are the first demonstration of MRI detection of targeted imaging by hyperpolarized silicon particles, and potentially opens the door to a variety of disease applications. The experiments were repeated (n=3) and compared to both chemical and biological controls (n=3 each), and histological analysis; tissue immunohistochemistry confirmed particle binding only under conditions of 214D4 particle conjugation and MUC1-expression. Furthermore, the long-lasting hyperpolarized 29Si signal was still well-visible for at least 15 minutes after injection. Future studies will translate these advances to nano-scale particles, which should demonstrate improved mobility and allow molecular targeting of other cancer systems.Conclusion
We demonstrate targeted molecular imaging of MUC1-expressing colorectal cancer in orthotopic mouse models using targeted silicon particles. When fully developed, these particles are engineered to be a platform system, where different targeting agents and therapeutic drugs can be attached for advanced molecular imaging and therapeutic interventions in the clinic.Acknowledgements
This work was funded by the MDACC Odyssey Postdoctoral Fellowship, NCI R25T CA057730, DoD PC131680, MDACC Institutional Research Grants, MDACC Institutional Startup, U54 CA151668, Leukemia and Brain SPORE Developmental Research Awards, NCI R21 CA185536, Gulf Coast Consortium, CPRIT RP150701, and NCI Cancer Center Support Grant CA016672.References
[1] SEER Cancer Statistics Review (2016)
[2] Soetikno, et al., JAMA (2008)
[3] Siegel, et al., JNCI (2017)
[4] Park, et al. Nat. Mat. (2009)
[5] Tasciotti, et al. Nat. Nano. (2008)
[6] Santos, Porous Silicon for Biomedical Applications, Elsiever (2014)
[7] Cassidy, et al. Nat. Nano. (2013)
[8] Dementyev, et al. Phys. Rev. Lett. (2008)
[9] Aptekar, et al. ACS Nano (2009)
[10] Whiting, et al. J. Med. Imag. (2016).
Figures