0098
Radiomics with magnetic resonance imaging of the breast for early prediction of response to neo-adjuvant chemotherapy in breast cancer patients1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Department of Scientific Computing, Florida State University, Tallahassee, FL, United States, 3Department of Biomedical Imaging and Image-guided Therapy, Medical University of Vienna, Vienna, Austria, 4Department of Pathology, Medical University of Vienna, Vienna, Austria
Synopsis
Breast cancer patients that achieve
Background and Aim
In patients undergoing neoadjuvant chemotherapy for breast cancer the achievement of a pathological complete response (pCR) is associated with a significantly improved disease-free and overall survival. Therefore, accurate means to predict treatment response as early as possible are desirable to identify women who don’t benefit from this cytotoxic therapy. Several studies have demonstrated that dynamic contrast-enhanced MRI is the most sensitive method for the assessment and prediction of treatment response (1-5). In the past decade, the field of medical image analysis has grown exponentially, with an increased number of pattern recognition tools and an increase in data set sizes. These advances have facilitated the development of processes for high-throughput extraction of quantitative features that result in the conversion of images into mineable data and the subsequent analysis of these data for decision support (6). This emerging field in medical research is termed radiomics. The aim of this study was to assess radiomics with multiparametric MRI using dynamic contrast-enhanced (DCE) MRI and T2-weighted for the early prediction of pCR in breast cancer patients undergoing neoadjuvant chemotherapy.Methods and materials
In this IRB approved prospective study 41 women (median age 50 years; range 25-80 years) with breast cancer scheduled for NAC were included and underwent state-of-the-art MRI of the breast at 3T with DCE and T2-weighted imaging prior to and after two cycles of NAC. For each lesion a total of 14 features were extracted ranging from morphological and kinetic MRI parameters. A recursive feature elimination method along with five different classifiers was performed including: linear support vector machine (SVM), linear discriminant analysis (LDA), logistic regression (LR), random forest (RF), and stochastic gradient descent (SGD) was employed to rank the features. Histopathology using the Residual Cancer Burden (RCB) score and class calculated from post-treatment surgical specimen and patient outcomes were used as the standard of reference.Results
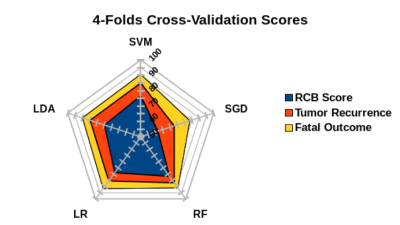
Classification accuracy was assessed for pCR as defined by the RCB score, metastases, and disease-specific death. Radiomics analysis of MRI data achieved AUCs for RCB score (AUC 0.85), metastases (AUC 0.87) based on RF and death (AUC 0.92) based on SVM. The most relevant parameters for prediction of RCB score were mass internal enhancement characteristics, shape, and margins with DCE, for metastasis peri-tumoral edema on T2-weighted imaging, mass margins and internal enhancement characteristics, and for death high signal intensity on T2-weighted imaging, mass margins, and internal enhancement characteristics (Fig 1 and 2).Conclusion
Radiomics with multiparametric MRI of the breast using DCE and T2-weighted imaging enables prediction of response to NAC with high accuracy and thus can provide predictive information to guide treatment decisions.
Acknowledgements
No acknowledgement found.References
1. Abramson RG, Li X, Hoyt TL, et al. Early assessment of breast cancer response to neoadjuvant chemotherapy by semi-quantitative analysis of high-temporal resolution DCE-MRI: preliminary results. Magn Reson Imaging. 2013;31(9):1457-64.
2. Arlinghaus LR, Li X, Levy M, et al. Current and future trends in magnetic resonance imaging assessments of the response of breast tumors to neoadjuvant chemotherapy. J Oncol. 2010;2010.
3. Li X, Abramson RG, Arlinghaus LR, et al. Multiparametric magnetic resonance imaging for predicting pathological response after the first cycle of neoadjuvant chemotherapy in breast cancer. Invest Radiol. 2015;50(4):195-204.
4. Wu LA, Chang RF, Huang CS, et al. Evaluation of the treatment response to neoadjuvant chemotherapy in locally advanced breast cancer using combined magnetic resonance vascular maps and apparent diffusion coefficient. J Magn Reson Imaging. 2015;42(5):1407-20.
5. Minarikova L, Bogner W, Pinker K, et al. Investigating the prediction value of multiparametric magnetic resonance imaging at 3 T in response to neoadjuvant chemotherapy in breast cancer. Eur Radiol. 2017;27(5):1901-11.
6. Gillies RJ, Kinahan PE, Hricak H. Radiomics: Images Are More than Pictures, They Are Data. Radiology. 2016;278(2):563-77.
Figures