0094
DCE-MRI based radiomics signature: a potential biomarker for preoperative prediction of sentinel lymph node metastasis in breast cancer1Biomedical Engineering, Stony Brook University, Stony Brook, NY, United States, 2Radiology, Guangdong General Hospital/Guangdong Academy of Medical Sciences, Guangzhou, China, 3Radiology, Stony Brook Medicine, Stony Brook, NY, United States, 4Computer Science, Stony Brook University, Stony Brook, NY, United States, 5Psychiatry, Stony Brook Medicine, Stony Brook, NY, United States
Synopsis
This study is the first to combine DCE-MRI radiomics with clinical information to predict sentinel lymph node (SLN) metastasis in breast cancer. The prediction model was established in a training set, and was further validated in a completely independent validation set, with AUC of 0.898 and negative predictive value of 0.902. This prediction performance surpasses the previous study using T2w and DWI, and is particularly useful for eliminating the unnecessary, invasive SLN biopsy and axillary dissection in patients with negative SLN, offering a step towards precision medicine of breast cancer.
Purpose
Breast cancer is the most commonly diagnosed cancer and second most common cause of cancer death among women in the U.S1. In particular, metastasis is the leading cause of mortality in breast cancer patients2. Sentinel lymph node (SLN) is the first station of lymph node metastasis in breast cancer, and thus SLN status offers a valuable prognostic factor to guide treatment decisions. Currently, SLN biopsy is the standard of care for nodal staging. In patients with negative SLN, axillary lymph node (ALN) metastasis is unlikely to happen and unnecessary invasive ALN dissection and its associated serious side effects can be avoided3. However, SLN biopsy is invasive and false negative rates over 10% have been reported4.
Radiomics is a technique of extracting high-dimensional quantitative features from medical images that enables the non-invasive identification of potential biomarkers for clinical-decision support5. A 2017 study used radiomics analysis to predict SLN metastasis based on T2-weighted fat suppression and diffusion-weighted MRI6. We hypothesize that dynamic contrast enhanced-MRI (DCE-MRI) combined with clinical information can provide more information on SLN and offer better preoperative predictors of SLN metastasis.
Methods
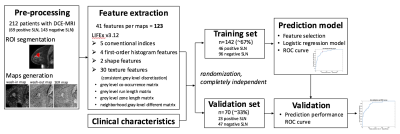
In this retrospective study with approved IRB exemption, a total of 212 patients (age: 56±12yr) with histologically proven breast cancer were identified. Sixty-nine patients were found to have histopathology-confirmed SLN metastasis and 143 were negative. DCE-MRI scans were performed on a 1.5T GE Signa HDxt using a sagittal VIBRANT multiphase sequence, including one pre-contrast and four post-contrast phases. Regions-of-interest (ROIs) containing enhancing tumor were drawn by an experienced breast radiologist on the first post-contrast images using MRIcron7. Imaging features were obtained from wash-in maps ((S1-S0)/S0), wash-out maps ((S1-S4)/S1) and signal enhancement ratio maps ((S1-S0)/(S4-S0)), where S0, S1, and S4 are the pre-contrast, first post-contrast and fourth post-contrast images, respectively. As shown in the workflow (Figure 1), forty-one features were extracted from each map using the same ROI in LIFEx v3.128, with consistent grey level discretization, leading to a total of 123 features for each patient. Clinical characteristics included patient age, number of lesions in the ipsilateral breast, histological grade, molecular subtypes and lymphovascular invasion status.
The dataset was randomly divided into two independent subsets: a training set (~67%, 142 patients) and a validation set (~33%, 70 patients). The training set was used for feature selection and predictive model generation. To avoid overfitting from redundant features, groups of highly correlated features (Spearman ρ≥0.95) were represented by the one with the largest intersubject variability. A backward selection method was used to identify significant features (t-test p-value<0.001 between positive and negative SLN). Combining clinical characteristics with the significant features, a logistic regression model was established in the training set. The receiver operating characteristic (ROC) curve was plotted, and the area under the ROC curve (AUC) was calculated. Optimal threshold was determined by maximizing the Youden index. The prediction model was further tested in the independent validation set using the determined threshold, with the ROC curve, AUC, sensitivity, specificity and negative predictive value (NPV) calculated in Matlab.
Results
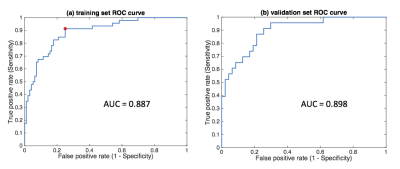
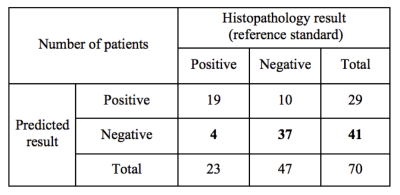
Using the training set, 15 variables were selected in the end for the logistic regression model (10 significant features with 5 clinical characteristics). Figure 2(a) shows the ROC curve of the training set (AUC=0.887, sensitivity=0.913, specificity=0.750). Figure 2(b) shows the corresponding result in the independent validation set (AUC=0.898, sensitivity=0.826, specificity=0.787). Table 1 demonstrates the model’s performance in predicting SLN metastasis in the validation set. For those patients predicted to have negative SLN, 37 out of 41 had a negative histopathology (NPV=0.902). The prediction performance (on the independent validation set) surpasses that of the previous study6 (AUC=0.805, sensitivity=0.700, specificity=0.747, NPV not reported) which used T2-weighted fat-suppression and diffusion-weighted MRI for feature extraction.Conclusion
This work is the first attempt to predict SLN metastasis by combining DCE-MRI radiomics and clinical information. The proposed method provides a potential non-invasive biomarker to preoperatively predict negative SLN with a high NPV of 0.902. This is particularly useful for eliminating unnecessary invasive SLN biopsy and ALN dissection. This study is a step towards precision medicine and personalized treatment for breast cancer patients. Our prediction model also outperforms the result of the previous study6, which used T2-weighted fat-suppression and diffusion-weighted MRI to predict SLN metastasis, although this could be due to different populations. Further studies are needed to validate the prediction performance on a larger dataset; we also expect the prediction performance to further improve by incorporating more advanced radiomics features.Acknowledgements
Carol M. Baldwin Breast Cancer Research Fund.References
1. Samson ME, Porter NG, Hurley DM, Adams SA, Eberth JM. Disparities in Breast Cancer Incidence, Mortality, and Quality of Care among African American and European American Women in South Carolina. Southern medical journal. 2016;109(1):24-30.
2. Ran S, Volk L, Hall K, Flister MJ. Lymphangiogenesis and lymphatic metastasis in breast cancer. Pathophysiology. 2010;17(4):229-251.
3. Fleming F, Kavanagh D, Crotty T, Quinn C, McDermott E, O’higgins N, Hill A. Factors affecting metastases to non-sentinel lymph nodes in breast cancer. Journal of clinical pathology. 2004;57(1):73-76.
4. Wong SL, Edwards MJ, Chao C, Tuttle TM, Noyes RD, Carlson DJ, Cerrito PB, McMasters KM. Sentinel lymph node biopsy for breast cancer: impact of the number of sentinel nodes removed on the false-negative rate. Journal of the American College of Surgeons. 2001;192(6):684-689.
5. Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology. 2015;278(2):563-577.
6. Dong Y, Feng Q, Yang W, Lu Z, Deng C, Zhang L, Lian Z, Liu J, Luo X, Pei S. Preoperative prediction of sentinel lymph node metastasis in breast cancer based on radiomics of T2-weighted fat-suppression and diffusion-weighted MRI. European Radiology. 2017:1-10.
7. Rorden C. MRICron [computer software]; 2007.
8. Nioche C, Orlhac F, Boughdad S, Reuze S, Soussan M, Robert C, Barakat C, Buvat I. A freeware for tumor heterogeneity characterization in PET, SPECT, CT, MRI and US to accelerate advances in radiomics. Journal of Nuclear Medicine. 2017;58(supplement 1):1316-1316.
Figures