0093
ACRIN 6702 DWI Trial: The Value of Alternate ADC Metrics Compared to Standard ADC for Decreasing Breast MRI False-Positives1Radiology, University of Washington, Seattle, WA, United States, 2ACRIN Biostatistics Center, Brown University, Providence, RI, United States, 3Memorial Sloan Kettering Cancer Center, New York, NY, United States
Synopsis
The ACRIN 6702 trial confirmed that standard ADCs from multi b-value DWI acquisition are lower in malignancies than in benign lesions and that application of standard ADCs can eliminate one in five unnecessary biopsies. Secondary analysis from this trial demonstrated that application of alternate ADC metrics, including perfusion insensitive ADCØ and normalized ADC provide no practical benefit over standard ADC for improving conventional DCE-MRI performance. These findings suggest that standard ADC calculations alone are sufficient for improving breast MRI specificity and should be the primary metric included in the next edition of the BI-RADS atlas.
Purpose
Breast MRI is the most sensitive modality for breast cancer detection and its utilization has increased over 20-fold in the last two decades (1). However, conventional dynamic contrast enhanced (DCE) based breast MRI approaches are limited by moderate specificity, resulting in many unnecessary biopsies. Multiple prior single-institution studies have demonstrated that incorporating diffusion weighted imaging (DWI) into breast MRI protocols may improve specificity, and initial results from the multicenter American College of Radiology Imaging Network (ACRIN) 6702 trial demonstrated that applying a threshold based on standard lesion ADC values to DCE-MRI BI-RADS assessments can reduce unnecessary biopsies by 21% (2). It has been proposed that alternate ADC metrics, including perfusion-insensitive ADC (ADCØ) computed with bmin>50 s/mm2 (3) and normalized ADC (ADCn) (4), could further improve breast MRI performance, but limited data exist to support their use. We thus aimed to assess the relative value of alternate ADC metrics versus standard ADCs to reduce breast MRI false-positives in ACRIN 6702.Materials and Methods
This single arm, prospective, multi-institution breast DWI trial accrued patients from March 2014 to April 2015. All potential participants provided written informed consent prior to undergoing a breast MRI with DCE and DWI sequences, and those with a BI-RADS category 3, 4, or 5 lesion identified on DCE-MRI only were enrolled. DCE-MRI sequences were performed in accordance with ACR-accreditation guidelines, and DWI was acquired prior to administration of contrast using a single shot diffusion-weighted spin-echo echo planar imaging (EPI) sequence with parallel imaging (reduction factor ≥ 2) and fat suppression. DWI (b-values = 0, 100, 600, and 800 s/mm2) was acquired axially (1.5-2.8 mm in-plane; 4-5 mm slice thickness) with diffusion gradients applied in three orthogonal directions. ADC values of all eligible BI-RADS category 3, 4, and 5 lesions were measured by the centralized core facility with researchers blinded to each lesion’s reference standard. In addition to standard ADC values (computed using all b-values), alternate ADC metrics of perfusion-insensitive ADCØ (using b = 100, 600, and 800 s/mm2 only), and ADCn (standard lesion ADC divided by normal fibroglandular tissue ADC) were measured for each lesion. Reference standard for all malignant lesions was pathology from biopsy, while benign lesions were confirmed with either biopsy or one year of negative clinical/imaging follow-up. Benign versus malignant mean standard ADC, ADCØ, and ADCn values were compared using bootstrapping, and combined performance of BI-RADS and ADC assessments w was described by calculating areas under Receiver Operator Characteristic (ROC) curves (AUCs) analyses.Results
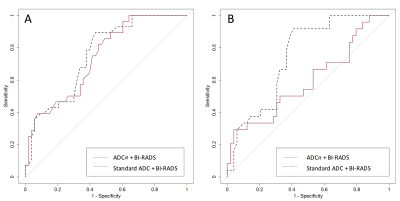
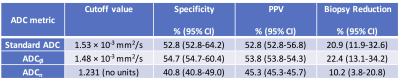
Of 1002 women screened at 10 participating sites, 106 eligible patients were identified and 103 (n=142 lesions) completed the study. Of those, 13 participants (n=28 lesions) were excluded due to missing reference standard and 23 (n=33 lesions) were excluded due to poor DWI quality precluding ADC measurements (5). The resulting analysis set comprised 67 patients (median age 49, range 24-75 years) with 81 BI-RADS category 3 (n=14), 4 (n=63), or 5 (n=4) lesions identified on DCE-MRI (28 malignant and 53 benign). Malignant lesions demonstrated significantly lower mean standard ADC (1.21 ± 0.21 vs. 1.47 ± 0.29 x 10-3 mm2/s) and ADCØ (1.17 ± 0.23 vs. 1.43 ± 0.29 x 10-3 mm2/s) than benign lesions (p<0.0001 for both, Figure 1). Mean ADCn also was lower in malignancies (0.83 ± 0.20 vs. 0.92 ± 0.26), though the difference was not significant (p=0.117). AUCs for predicting malignancy when combined with BI-RADS was 0.75 for standard ADC (95% CI =0.65-0.84), 0.74 for ADCØ (95% CI =0.63-0.83), and 0.60 for ADCn (95% CI =0.40-0.76) (Figure 2). Cutoff values identified for each of the three metrics to maintain 100% sensitivity and achieve maximal specificity were 1.53 × 10-3 mm2/s for standard ADC, 1.48 × 10-3 mm2/s for ADCØ, and 1.231 for ADCn, resulting in 20.9%, 22.4%, and 10.2% reductions in unnecessary biopsies, respectively (Table 1). Applying these cutoffs resulted in combined DCE BI-RADS plus DWI ADC breast MRI specificity of 52.8% using standard ADC, 54.7% using ADCØ, and 40.8% using ADCn.Conclusion
Secondary analysis from this multicenter breast DWI trial demonstrates that alternate ADC metrics provide little additional value over standard ADCs for increasing breast MRI specificity without lowering sensitivity. Specifically, ADCn did not demonstrate significant differences between malignant and benign lesions while ADCØ provided similar performance benefit to standard ADC when combined with DCE-MRI BI-RADS assessments, with only marginally greater biopsy reduction and specificity. These findings suggest that computing ADCs to eliminate microperfusion effects or normalize ADCs to lesion-free fibroglandular tissue is unlikely to further reduce breast MRI false-positives than standard ADCs.Acknowledgements
This research was supported by the American College of Radiology Imaging Network, ACRIN, which receives funding from the National Cancer Institute (NCI) through the grants U01 CA079778 and U01 CA080098. Performance of central ADC measures was supported by NIH/NCI R01CA151326 (P.I. Partridge).References
1. Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Intern Med 2014;174(1):114-121.
2. Partridge SC ZZ, Rahbar H, Chenevert TL, Kitsch AE, Hanna L, Romanoff J, Harvey S, Moy L, DeMartini W, Schnall M, Lehman CD, Comstock C. ACRIN 6702 Trial: A MultiCenter Study Evaluating the Utility of Diffusion Weighted Imaging for Detection and Diagnosis of Breast Cancer. Accepted for presentation at RSNA, Chicago, IL, Nov 2017.
3. Bogner W, Gruber S, Pinker K, et al. Diffusion-weighted MR for differentiation of breast lesions at 3.0 T: how does selection of diffusion protocols affect diagnosis? Radiology 2009;253(2):341-351.
4. El Khouli RH, Jacobs MA, Mezban SD, et al. Diffusion-weighted imaging improves the diagnostic accuracy of conventional 3.0-T breast MR imaging. Radiology 2010;256(1):64-73.
5. Kitsch A ZZ, Chenevert TL, Rahbar H, Romanoff J, Whisenant J, Yankeelov T, Comstock C, Partridge SC. American College of Radiology Imaging Network (ACRIN) 6702 Diffusion-Weighted Breast MRI Trial: Image Quality and Factors Associated with Lesion Evaluability. Accepted for presentation at RSNA, Chicago, IL, Nov 2017.
Figures