0089
The Age effect on Multi-parametric Magnetic Resonance Imaging changes in Multiple Sclerosis lesions1Martinos Center for Biomedical Imaging, Massachusetts General Hospital and Harvard Medical School, Boston, MA, United States, 2Signal Processing Laboratory (LTS 5), École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 3Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 4Department of Radiology, Centre Hospitalier Universitaire Vaudois (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Neurology Department and Neuroimaging Laboratory, Basel University Hospital, Basel, Switzerland
Synopsis
We assessed the effect of age on the longitudinal evolution of intralesional neurite density and orientation dispersion indices, magnetization transfer ratio and T1 relaxometry in a cohort of relapsing-remitting MS patients. While we observed a decrease of neurite dispersion in lesions and stable neurite density, MTR and qT1, age did not seem to influence those longitudinal changes in MS lesions.
Introduction
Aging is known to modify the characteristics of brain morphology and microstructure in both physiological and pathological conditions1,2,3. Specifically, older age has been associated with less brain plasticity4 and less efficient repair mechanisms following a focal or diffuse brain pathology5. Recently, we have shown that a combination of MRI parameters derived from “neurite density and orientation dispersion imaging” (NODDI) on diffusion MRI, Magnetization Transfer imaging and T1 relaxometry permits to identify a microstructural pattern reflecting with brain repair in MS patients6. However, it is still unclear whether patients' age influences the observed longitudinal parametric changes.Material and Methods
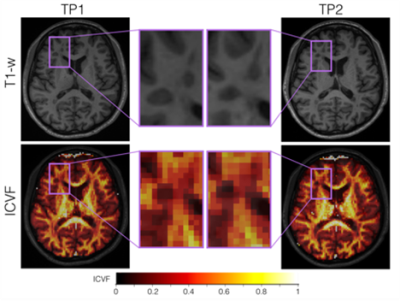
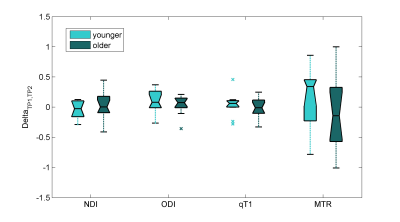
We studied 30 RRMS patients on therapy [10 males, age (standard deviation) 34.6 (8.4) years] with less than 5 years from initial symptoms. 3T MR images were acquired on a MAGNETOM Trio a Tim system (Siemens Healthcare, Erlangen, Germany) at baseline and 2-years follow-up. MS lesions were manually segmented by two raters by consensus on 3D DIR, 3D MP2RAGE, and 3D FLAIR images. A union mask of the lesions segmented in each contrast was then obtained. Subsequently, we derived the Neurite Density Index (NDI) and the Orientation Dispersion Index (ODI), T1 and MTR voxel-wise maps from the diffusion, relaxometry, and magnetization transfer acquisitions. Paired t-tests and Bonferroni correction for multiple comparison were used to analyze the evolution of tissue microstructure within white matter (WM) MS lesions, which were pooled into: (i) enlarged (n=8), if their volume at time-point 2 (TP2) was the twice of the volume as at baseline, (ii) shrunken (n=8), if the volume was decreased by 50% at TP2, and (iii) stable (n=42), otherwise7. We then focused on the group of lesions that showed longitudinal parametric differences (stable lesions) and evaluated the age effect by selecting two sub-cohorts of patients: i) age below the 25th percentile of our sample (young patients), ii) age higher than the 75th percentile of our sample (older patients). Paired t-tests were carried out between the normalized delta (TP1-TP2)/(TP1) of each MRI parameter (NDI, ODI, qT1 and MTR) in young and old patients. A linear mixed-effects model per each MRI parameter was applied using age and gender as predictors, and normalized delta between TP1 and TP2 of MRI metrics as outcome.Results
Figure 1 shows the longitudinal decrease on ODI (Pcorrected<0.05) over two years where NDI, qT1 and MTR appeared not to be significant. Pair-wise t-test of normalized delta values between young and older patients did not show any significant difference (Figure 2, Pcorrected>0.26). No significant associations between age or gender and normalized delta for each MRI parameter were found using the liner mixed effects model (Pcorrected>0.7).Discussion and Conlusion
Our study suggests that age does not influence the repair pattern measured longitudinally in MS lesions of RRMS patients by applying multi-parametric MRI (NODDI, T1 relaxometry and MTI). Future studies should confirm our findings in larger and more heterogeneous MS patient cohorts.Acknowledgements
No acknowledgement found.References
1- Fjell AM, Walhovd KB. Structural brain changes in aging: courses, causes and cognitive consequences. Rev Neurosci. 2010;21(3):187-221.
2- Imhof A, Kövari E, von Gunten A, Gold G, Rivara CB, Herrmann FR, Hof PR, Bouras C, Giannakopoulos P. Morphological substrates of cognitive decline in nonagenarians and centenarians: a new paradigm?. J Neurol Sci. 2007 Jun 15;257(1-2):72-9
3- Salvadores N, Sanhueza M, Manque P, Court A.F. Axonal Degeneration during Aging and Its Functional Role in Neurodegenerative Disorders. Front Neurol 2017;
4- McLaughlin CN, Broihier HT. Keeping Neurons Young and Foxy: FoxOs Promote Neuronal Plasticity. Trends Genet 2017;
5- Filippi M, Preziosa P, Rocca MA. Brain mapping in multiple sclerosis: Lessons learned about the human brain. Neuroimage. S1053-8119(17)30769-3;
6- Fischi E, Bommier G, Romascano D, Daducci A, Setsompop K, Thiran J-P, Krueger G, Granziera C. Longitudinal study of MS lesion evolution using neuritis orientation dispersion and density imaging, relaxometry and magnetization transfer imaging. ECTRIMS 2017;
7- Moraal B, Wattjes MP, Geurts JJ, Knol DL, van Schijndel RA, Pouwels PJ, et al. Improved Detection of Active Multiple Sclerosis Lesions: 3D Subtraction Imaging . Radiology. 2010 Apr;255(1):154-63.
Figures