0087
A 3-year follow-up study of enhancing and non-enhancing multiple sclerosis (MS) lesions in MS patients with clinically isolated syndrome (CIS) using a multi-compartment T2 relaxometry (MCT2) model1University of Rennes, INRIA, CNRS, INSERM, IRISA UMR 6074, VISAGES ERL U-1228, F-35000 Rennes, France, Rennes, France, 2CRL, Boston Children’s Hospital, Department of Radiology, 300 Longwood Avenue, WB215, Boston, MA 02115, USA, Boston, MA, United States, 3Department of Neurology, Rennes University Hospital, Rennes, France, Rennes, France
Synopsis
Obtaining information on condition of tissue microstructures (such as myelin, intra/extra cellular cells, free water) can provide important insights into MS lesions. However, MRI voxels are heterogeneous in terms of tissue microstructure due to the limited imaging resolution owing to existing physical limitations of MRI scanners. Here we evaluated a multi-compartment T2 relaxometry model and then used it to study the evolution of enhancing (USPIO and gadolinium positive) and non-enhancing lesions in 6 MS patients with CIS characteristics over a period 3 years with 7 follow-up scans after baseline.
Introduction
Demyelination occurs at the onset of MS accompanied by macrophage intervention1,2. This is followed by axonal damage. Continuous tissue injuries cause inflammation in lesion affected regions of the brain. Advanced MRI techniques such as T2 relaxometry and diffusion MR sequences can help in obtaining information on brain tissue microstructure. Myelin, intra/extra-cellular fluids and free water can be distinguished by their different T2 relaxation times3. Myelin has a very short T2 relaxation time3 and its presence in WM is quantified by a variety of approaches3-7. The most popular approach is to obtain the myelin water fraction (MWF) in a tissue3-7 from a multi-echo spin echo (SE) T2 sequence. However, since MWF is a relative measure, a decrease or increase in it only conveys part of the information. In this work, we use a multi-compartment T2 (MCT2) relaxometry model8 to obtain water fraction (WF) corresponding to tissues with short, medium and high T2 relaxation times. We perform test-retest experiments on 4 healthy controls (HC) to demonstrate the reproducibility of the WF estimates obtained from this model. A 3-year follow-up study is performed on 6 MS patients demonstrating clinically isolated syndrome (CIS). We study the evolution of WF in enhancing and non-enhancing MS lesions in white matter (WM).Method and materials
MCT2 model:
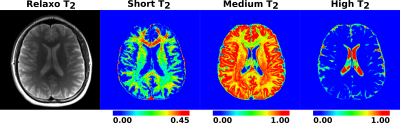
In the MCT2 model8, the T2 space is modeled as a weighted mixture of three continuous Gaussian probability density functions representing short, medium and high decaying components with respect to their T2 relaxation times. The short T2 WF (WFshort) provides information on myelin3 and highly myelinated axons10 in WM. The medium T2 WF (WFmedium) corresponds to the intra/extra-cellular matters3,10. The high T2 WF (WFhigh) represents cerebrospinal fluid and inflammation in WM due to lesions.
Reproducibility study:
Test-retest scans were performed for 4 HC. Average values in 8 WM regions were computed. A Bland-Altman plot was observed for assessing the reproducibility of the WF estimates. Similar to a previous study13 we used this approach to obtain the reproducibility threshold value of WF estimates.
T2 relaxometry data: 3T MRI scanner, first echo time (TE)=9ms, echo spacing (∆TE)=9ms repetition time (TR)=2030ms, number of echoes (nechoes)=32, voxel dimensions (vd)=1.64x1.64x4mm3. All images were registered11,12 to a common T2-weighted image.
MS lesion study:
Six MS patients demonstrating CIS condition were scanned at baseline and months {3,6,9,12,18,24,36}. The median age of patients included in this study was 28.5 years at baseline and there was an equal number of male and female subjects.
T2 relaxometry data: 3T scanner, first TE=13.8ms, ∆TE=13.8ms, TR=4530ms, nechoes=7, vd=1.3x1.3x3mm3, acquisition time=7minutes.
For months-{0,3,6,9}: transverse SE T1-weighted images (1x1x3mm3) were acquired before and after USPIO infusion (SHU-555C;40µmol Fe/kg body weight over 30minutes) for obtaining USPIO enhanced lesions. A scan was done 24 hours later to obtain post-USPIO enhancement images.
Transverse SE T1-weighted images (1x1x3mm3) post gadolinium contrast agent infusion (0.1mmol/kg gadopentetate dimeglumine) were acquired to find gadolinium enhanced lesions.
Following lesion types14 were studied:
- (U+): Appear on post-contrast USPIO and Gadolinium scans.
- (Gd+): Appears on post-contrast Gadolinium scan, but not in (U+).
- (L-): MS lesion ROI not in (Gd+) and (U+).
- (E+): Enhancing lesions, i.e. (Gd+)$$$\;\cup\;$$$(U+)
We analyzed 111(L-), 23(Gd+) and 6(U+) lesions. For each type we studied the percentage of lesions that underwent changes above the reproducibility threshold between an acquisition and follow-up, and the evolution of MCT2 WF estimates over a period of 36 months.
All images were registered to the 3D FLAIR image (1x1x1mm3) acquired at baseline.
The protocols were approved by the institutional review board of Rennes University Hospital, and all participants gave their written consent.
Results and discussions
Figure-1 shows WF maps of a healthy control.
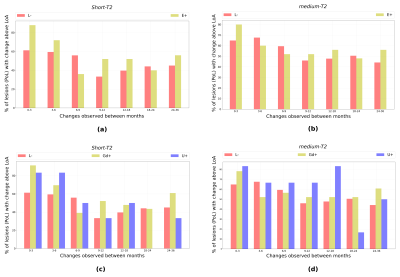
The reproducibility results (refer Figure-2) show that the 95% limits of agreement (LoA) for both WFshort and WFmedium are 0.013. The percentage of lesions (PoL) undergoing change in WFshort above LoA (refer Figure-3(a)) are relatively smaller for (L-) than (E+) lesions.
The PoL undergoing change in WFshort (refer Figure-3(a) and 3(c)) above LoA reduces after 6 months. In general, (L-) have lesser PoL undergoing change above LoA as compared to (Gd+) and (U+).
The evolution of WF in each lesion type is shown in Figure-4.
Conclusion
The MCT28 estimates are reproducible and provided insights into the lesion that corroborate with MS lesion pathological studies2. (U+) lesions showed higher variation in initial months as compared to (Gd+) and (L-). By the end of month-36, (L-), (U+) and (Gd+) attain similar WFshort and WFhigh values. We now intend to perform more rigorous statistical analysis to evaluate whether MCT2 estimates can significantly differentiate the lesion groups studied in this abstract.Acknowledgements
MRI data acquisition was supported by the Neurinfo MRI research facility from the University of Rennes I. Neurinfo is granted by the the European Union (FEDER), the French State, the Brittany Council, Rennes Metropole, Inria, Inserm and the University Hospital of Rennes. This research was also supported in part by the grants NIH R01 EB019483 and NIH R01 NS079788.References
[1] Barillot, Christian, Gilles Edan, and Olivier Commowick. "Imaging biomarkers in Multiple Sclerosis: from image analysis to population imaging." (2016): 134-139.
[2] Lassmann, Hans, Wolfgang Brück, and Claudia Lucchinetti. "Heterogeneity of multiple sclerosis pathogenesis: implications for diagnosis and therapy." Trends in molecular medicine 7, no. 3 (2001): 115-121.
[3] MacKay, Alex, Cornelia Laule, Irene Vavasour, Thorarin Bjarnason, Shannon Kolind, and Burkhard Mädler. "Insights into brain microstructure from the T 2 distribution." Magnetic resonance imaging 24, no. 4 (2006): 515-525.
[4] Whittall, Kenneth P., Alex L. Mackay, Douglas A. Graeb, Robert A. Nugent, David KB Li, and Donald W. Paty. "In vivo measurement of T2 distributions and water contents in normal human brain." Magnetic resonance in medicine 37, no. 1 (1997): 34-43.
[5] Laule, C., E. Leung, D. KB Li, A. L. Traboulsee, D. W. Paty, A. L. MacKay, and G. RW Moore. "Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology." Multiple Sclerosis Journal 12, no. 6 (2006): 747-753.
[6] MacKay, Alex L., and Cornelia Laule. "Magnetic Resonance of Myelin Water: An in vivo Marker for Myelin." Brain Plasticity 2, no. 1 (2016): 71-91.
[7] Akhondi‐Asl, Alireza, Onur Afacan, Mukund Balasubramanian, Robert V. Mulkern, and Simon K. Warfield. "Fast myelin water fraction estimation using 2D multislice CPMG." Magnetic resonance in medicine 76, no. 4 (2016): 1301-1313.
[8] Chatterjee, Sudhanya, Olivier Commowick, Simon Warfield, and Christian Barillot. "Gaining Insights Into Multiple Sclerosis Lesion Characteristics from Brain Tissue Microstructure Information: A Multi-Compartment T2 Relaxometry Approach." In ISMRM 25TH ANNUAL MEETING & EXHIBITION. 2017.
[9] Prasloski, Thomas, Burkhard Mädler, Qing‐San Xiang, Alex MacKay, and Craig Jones. "Applications of stimulated echo correction to multicomponent T2 analysis." Magnetic resonance in medicine 67, no. 6 (2012): 1803-1814.
[10] Lancaster, Jack L., Trevor Andrews, L. Jean Hardies, Stephen Dodd, and Peter T. Fox. "Three‐pool model of white matter." Journal of Magnetic Resonance Imaging 17, no. 1 (2003): 1-10.
[11] Commowick, Olivier, Nicolas Wiest-Daesslé, and Sylvain Prima. "Block-matching strategies for rigid registration of multimodal medical images." In Biomedical Imaging (ISBI), 2012 9th IEEE International Symposium on, pp. 700-703. IEEE, 2012.
[12] Ourselin, Sébastien, Alexis Roche, Sylvain Prima, and Nicholas Ayache. "Block matching: A general framework to improve robustness of rigid registration of medical images." In MICCAI, vol. 1935, pp. 557-566. 2000.
[13] Vargas, Wendy S., Elizabeth Monohan, Sneha Pandya, Ashish Raj, Timothy Vartanian, Thanh D. Nguyen, Sandra M. Hurtado Rúa, and Susan A. Gauthier. "Measuring longitudinal myelin water fraction in new multiple sclerosis lesions." NeuroImage: Clinical 9 (2015): 369-375.
[14] Kerbrat, Anne, Benoit Combès, Olivier Commowick, Adil Maarouf, Elise Bannier, Jean Christophe Ferré, Ayman Tourbah, Jean-Philippe Ranjeva, Christian Barillot, and Gilles Edan. "USPIO-positive MS lesions are associated with greater tissue damage than gadolinium-positive-only lesions during 3-year follow-up." Multiple Sclerosis Journal (2017): 1352458517736148.
Figures