0081
Using Tumor Stiffness as a Potential Biomarker for Predicting Hepatocellular Carcinoma Recurrence1Department of Radiology, the Third Affiliated Hospital of Sun Yat-sen University(SYSU), Guangzhou, China, 2Department of Pathology, the Third Affiliated Hospital of Sun Yat-sen University(SYSU), Guangzhou, China, 3Department of Radiology, Mayo Clinic, Rochester, MN, United States, 4Department of Epidemiology and Biostatistics, West China School of Public Health Sichuan University, ChengDu, China
Synopsis
Hepatocellular carcinoma (HCC) is a highly aggressive cancer and one of the leading causes of cancer-related deaths around the world. Our preliminary analysis of 78HCCsshowed 3D MRE is a promising, noninvasive technique for predicting the early recurrence of HCCs after hepatic resection. MRE-assessed tumor stiffness correlates with features such as encapsulation, macrovascular invasion, and histological grade. In the future, larger studies will improve our understanding of the relationship between HCC stiffness, invasiveness, and outcome for better allocation of treatment strategies and surveillance follow-up.
Introduction
Hepatocellular carcinoma (HCC) has a high rate of recurrence after surgery and, therefore, preoperative knowledge of histological features is crucial for the management of HCC patients1-5. Increased tumor stiffness is associated with tumor invasion and metastasis6-8. Magnetic resonance elastography (MRE) can measure the tumor stiffness noninvasively9-12. We investigated whether MRE could assess the pathologic features of HCC, including vascular invasion, capsule tumor formation, and histological differentiation, and preoperatively predict early recurrence after surgery.Methods
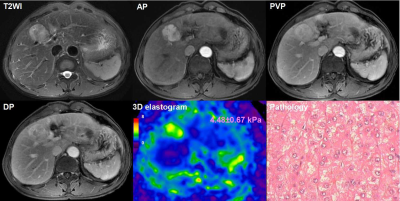
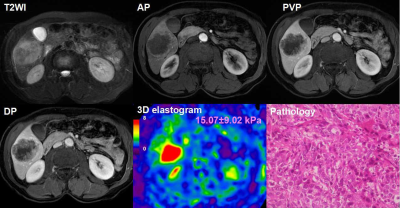
We performed a retrospective study of 78 patients(69 males and 9 females) who, between December 2014 and August 2017, underwent hepatic resection for HCC and received a preoperative MRE examination within one month of surgery. Histology specimens were reviewed according to WHO criteria for grade, capsule, vascular invasion, and surgical resection margin (invasion or free of tumor) by an experienced hepatopathologist who was blinded to the clinical data and MRE results13. Fibrosis staging of the liver parenchyma was also performed. MRE was performed on a 3.0T scanner with a SE-based, multislice, single-shot, EPI, 3D-MRE sequence at 60Hz (acquisition matrix: 80x80; TR/TE:1334/52ms; scan time:1:04 min; FOV:44.8 cm; number of slices: 20; slice thickness: 3mm).The MRE magnitude and phase images were processed using a 3D direct inversion (DI) of the Helmholtz wave equation using the curl of the MRE-measured wave field to calculate the stiffness. In patients with multiple tumors, the largest one was analyzed. Regions of interest(ROIs) were drawn by manually tracing the HCC on every slice demonstrating the tumor and covering the entire HCC on the MRE magnitude images usingT2-weighted images as a reference. The ROIs excluded the tumor edges, areas of significant wave interference, and any other artifacts seen on the magnitude and phase images. The mean HCC stiffness from the ROI was reported in kilopascals (kPa). The correlation between the histological features and the stiffness of the tumor were assessed using univariate and multiple linear regression analysis. The univariate and multivariate Cox proportional hazard model were used to evaluate risk factors associated with early recurrence after surgery. Early-recurrence curves were computed using the Kaplan–Meier method and were compared using the log–rank test.Results
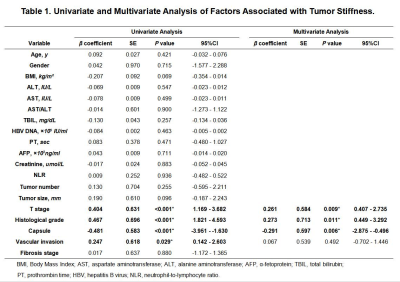
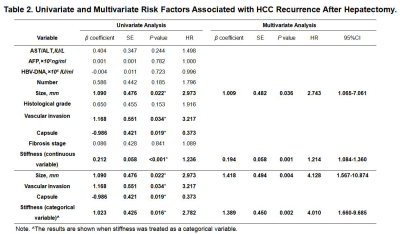
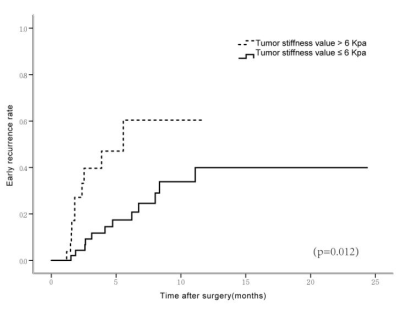
The majority of patients (58/78, 74.0%) had a single HCC. The size of the tumor ranged from 26 to 126mm, with a mean± SD diameter of 59.31±26.10mm. Forty tumors were >5 cm in diameter, 31were 3-5cm, and 7 were<3 cm.53 HCCs (68.0%) had capsule and 25(32.0%) did not have any capsule. Tumor grade was well differentiated in 10cases (12.8%), moderately differentiated in 53(67.9%), and poorly differentiated in 15(19.2%). 48(62%) tumors had vascular invasion, and 11 of them had macrovascular invasion on MR imaging or gross examination. Tumor T stage (β=0.261, P=0.009), capsule (β=-0.291, P=0.006) and histological grade (β=0.273, P=0.011) were significantly associated with tumor stiffness(Table1). Tumor size(HR=2.743; 95% CI: [1.065, 7.061], P= 0.036) and stiffness treated as a continuous variable (HR=1.214; 95% CI: [1.084, 1.360], P= 0.001), and tumor size (HR=4.128; 95% CI: [1.567, 10.874], P= 0.004) and stiffness dichotomized using a cutoff of 6 kPa (HR=4.010; 95% CI: [1.660, 9.685], P= 0.002) were risk predictors of early recurrence(Table2). Tumors with a mean stiffness >6 kPa showed a significantly higher early recurrence rate compared to those ≤6 kPa (39%,11/28 vs. 26%, 13/50, P =0.012)(Figure 1,Figure 2, Figure 3).Discussion
The results of this study show that poorly differentiated HCCs, HCCs with no capsule formation, and HCCs with advanced T stage were significantly stiffer than HCCs that were well or moderately differentiated, had a capsule, and had low T stage. Tumor stiffness was also independently associated with histological grade, capsule formation, and T stage. Tumor size and stiffness were found to be risk factors for early recurrence after surgery in patients with HCC. Tumors with a mean stiffness >6 kPa showed a significantly higher early recurrence rate compared to those ≤6 kPa. These preliminary results suggest that MRE is a potential noninvasive imaging biomarker for predicting histological results and early recurrence of HCCs.Conclusion
HCC tumor stiffness may be a useful noninvasive quantitative biomarker for the prediction of pathologic features and early recurrence after hepatic resection.Acknowledgements
No acknowledgement found.References
1. de Martel C, Maucort-Boulch D, Plummer M, et al. World-wide relative contribution of hepatitis B and C viruses in hepatocellular carcinoma. Hepatology. 2015; 62:1190-200.
2. Roayaie S, Blume IN, Thung SN, et al. A system of classifying microvascular invasion to predict outcome after resection in patients with hepatocellular carcinoma. Gastroenterology. 2009; 137:850-5.
3. Sapisochin G, Castells L, Dopazo C, et al. Single HCC in cirrhotic patients: liver resection or liver transplantation? Long-term outcome according to an intention-to-treat basis. Ann Surg Oncol. 2013; 20:1194-202.
4. Vitale A, Morales RR, Zanus G, et al. Barcelona Clinic Liver Cancer staging and transplant survival benefit for patients with hepatocellular carcinoma: a multicentre, cohort study. Lancet Oncol. 2011; 12:654-62.
5. Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012; 379:1245-55.
6. Wong CC, Tse AP, Huang YP, et al. Lysyl oxidase-like 2 is critical to tumor microenvironment and metastatic niche formation in hepatocellular carcinoma. Hepatology. 2014; 60:1645-58.
7. Schrader J, Gordon-Walker TT, Aucott RL, et al. Matrix stiffness modulates proliferation, chemotherapeutic response, and dormancy in hepatocellular carcinoma cells. Hepatology. 2011; 53:1192-205.
8. Pang M, Teng Y, Huang J, et al. Substrate stiffness promotes latent TGF-beta1 activation in hepatocellular carcinoma. Biochem Biophys Res Commun. 2017; 483:553-8.
9. Lee DH, Lee JM, Yi NJ, et al. Hepatic stiffness measurement by using MR elastography: prognostic values after hepatic resection for hepatocellular carcinoma. Eur Radiol. 2017; 27:1713-21.
10. Venkatesh SK, Yin M, Glockner JF, et al. MR elastography of liver tumors: preliminary results. AJR Am J Roentgenol. 2008; 190:1534-40.
11. Thompson SM, Wang J, Chandan VS, et al. MR elastography of hepatocellular carcinoma: Correlation of tumor stiffness with histopathology features-Preliminary findings. Magn Reson Imaging. 2016; 37:41-5.
12. Wang J, Yang H, Liu Y, et al. 3D MR Elastograpy in Prediction of Tumor Capsule Formation of Hepatocellular Carcinoma (HCC) in Patients with Hepatitis B Virus Infection. ISMRM, Hawaii, 2017, Number: 6187
13. Aaltonen LA, Hamilton SR, World Health Organization., et al. Pathology and genetics of tumours of the digestive system. Lyon Oxford: IARC Press ; Oxford University Press (distributor. 314p.
Figures