0074
Prospective Comparison of Gadoxetic Acid-Enhanced Liver MRI And Contrast-Enhanced CT With Histopathological Correlation For Preoperative Detection Of Colorectal Liver Metastases Following Chemotherapy And Potential Impact On Surgical PlanKartik Jhaveri1, Sandra Fischer1, Hooman Hosseini Nik1, Ravi Menezes1, Steven Gallinger1, and Carol-Ann Moulton1
1UHN, Toronto, ON, Canada
Synopsis
Complete resection of colorectal cancer liver metastases increases survival and is a recommended therapeutic option. Thus accurate detection of liver metastases is crucial. Many patients receive preoperative chemotherapy which often causes hepatic steatosis and decreases sensitivity of CT in detecting liver metastases. This prospective study with histopathological correlation compared the diagnostic performance of gadoxetic acid Liver MRI in the preoperative detection of liver metastases following chemotherapy including the influence of hepatic steatosis and lesion size. We also evaluated the potential change in the hepatic resection plan due to inclusion of gadoxetic acid MRI compared to CT.
Introduction:
Complete resection of colorectal liver metastases(CRLM) can achieve 5-year and 10-year survival rates of up to 45% and 25% respectively and currently recommended under NCCN guidelines. However, majority of patients with CRLM including initially unresectable CRLM receive preoperative chemotherapy with downstaging intent. In chemonaive patients, gadoxetic acid-enhanced MRI (EOB-MRI) has shown higher sensitivity in the preoperative detection of CRLM compared to both contrast enhanced CT(CECT) and extracellular contrast-enhanced MRI. Hepatic steatosis following chemotherapy can decrease the sensitivity of CT with implications towards inadequate liver resection. CECT is still commonly utilized as the primary tool for detection of liver metastases as well as surgical planning, because of its availability and lower relative cost.EOB-MRI in the post-chemotherapy scenario has been investigated in only a few mostly retrospective studies.Purpose:
To prospectively compare the diagnostic performance of EOB-MRI and CECT for preoperative detection of CRLM following chemotherapy and potential change in the hepatic resection plan with EOB-MRI vs CECT.Methods:
51 patients with CRLM treated with preoperative chemotherapy underwent liver imaging by EOB-MRI and CECT in this single-center prospective HIPAA compliant study with written informed consent. CT scans were performed with a 64-MDCTscanner (Aquilion 64, Toshiba America Medical Systems, CA, USA) using a portal venous phase protocol. Scanner parameters were detector configuration, 64 × 0.5 (32 mm); tube rotation speed, 0.5 s; tube voltage, 120 kV; automated tube current of 50–400 mA, and exposure time of 400–750 ms. All EOB-MRI were performed on the same 3T MRI scanner (Verio, Siemens Healthcare, Erlangen, Germany) using a standard liver MRI protocol with gadoxetic acid including diffusion weighted imaging (DWI) including b-value up to 800 s/mm2 and dynamic contrast-enhanced with 20-minute hepatobiliary phase imaging. Gadoxetic acid (Primovist or Eovist, Bayer AG, Germany) was administered intravenously with a power injector at a rate of 1 mL/s (0.025 mmol/kg BW). Two blinded subspecialty abdominal radiologists, with 15 years and 5 years of experience in CT and MRI, independently reviewed the anonymized images with a gap of more than four weeks between the two modalities to minimize recall probability. Surgical treatment was aimed at resecting all the detected CRLM on pre-operative imaging, with the interval between imaging and surgery planned to be less than six weeks. The surgical specimens were evaluated histopathologically for diagnostic confirmation.41 patients underwent hepatic resection and histopathological evaluation.Results:
151 CRLM were confirmed by histology. Mean lesion diameter was 2.0 cm on CT (range, 0.2‒10.5 cm) and 1.5 cm on MRI (range, 0.2‒10.5 cm) for the reader 1; and 1.7 cm on CT (range, 0.2‒9.3 cm) and 1.6 cm on MRI (range, 0.2‒11.0 cm) for the reader 2.With histology as reference standard, EOB-MRI, compared to CECT, had significantly higher sensitivity in detection of CRLM 1.0 cm (86% vs. 45.5%; p < 0.001), significantly lower indeterminate lesions diagnosis (7% vs. 33%; p < 0.001) and significantly higher interobserver concordance rate in characterizing the lesions 1.0 cm (72% vs. 51%; p = 0.041). MRI detected additional metastases (41 and 38 lesions for reader 1 and 2) compared to CT. For both readers, the proportion of CRLM ≤ 1.0 cm among the additional metastases was significantly higher than that among those seen on both modalities. The incidence of additional metastases (diagnosed only on MRI) was significantly higher in livers with steatosis compared to those without steatosis (47% vs. 22%, p = 0.005). Hypothetically, if liver resection plans had been drawn individually by CECT and EOB-MRI, the surgical could have changed the surgical plan in 45% of patients due to increased yield in CRLM detection.Discussion:
A significant proportion of hepatic lesions in patients with colorectal cancer are small in size which impose diagnostic and hepatic resection planning challenges.There was a positive association between lesion size of ≤ 1.0 cm and the presence of hepatic steatosis in the group of metastases seen only on MRI. In addition, EOB-MRI showed greater diagnostic confidence with significantly lower incidence of indeterminate lesions.There was increased yield with EOB-MRI in detecting CRLM at an organ, lobar and or segmental level compared to CT.The potential change in surgical plan with EOB-MRI of almost half of the cohort is significantly higher in the post chemotherapy scenario compared to prior studies assessing only chemonaive cohorts.Conclusion:
Following preoperative chemotherapy, EOB-MRI is superior to CECT in detection of small CRLM (<1 cm) with significantly higher sensitivity, diagnostic confidence and interobserver concordance in lesion characterization. EOB-MRI can alter the surgical plan in almost half of patients scheduled for liver resection versus CECT.Acknowledgements
This study has received research funding from Bayer AG, Germany.References
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FSet al. (2012) Survival after liver resection in metastatic colorectal cancer:review and meta-analysis of prognostic factors. Clin Epidemiol 4:283–301.
- NCCN. (2016) Clinical practice guidelines for treatment of colon cancer.Version 2. National Comprehensive Cancer Network (NCCN) [updated24 Nov 2015; cited 2016 Feb]. Available from: http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- Jhaveri K, Cleary S, Audet P, Balaa F, Bhayana D, Burak K et al. (2015)Consensus statements from a multidisciplinary expert panel on theutilization and application of a liver-specific MRI contrast agent(gadoxetic acid). Am J Roentgenol 204:498–509.
- van Kessel CS, Buckens CF, van den Bosch MA, van Leeuwen MS, vanHillegersberg R, Verkooijen HM. (2012) Preoperative imaging of colorectalliver metastases after neoadjuvant chemotherapy: a meta-analysis.Ann Surg Oncol 19:2805–2813.
- Kulemann V, Schima W, Tamandl D, Kaczirek K, Gruenberger T, Wrba Fet al. (2011) Preoperative detection of colorectal liver metastases in fattyliver: MDCT or MRI? Eur J Radiol 79:e1–6.
- Zech CJ, Korpraphong P, Huppertz A, Denecke T, Kim MJ,Tanomkiat W et al. (2014) Randomized multicentre trial of gadoxeticacid-enhanced MRI versus conventional MRI or CT in the staging ofcolorectal cancer liver metastases.
- Berger-Kulemann V, Schima W, Baroud S, Koelblinger C, Kaczirek K,Gruenberger T et al. (2012) Gadoxetic acid-enhanced 3.0 T MR imagingversus multidetector-row CT in the detection of colorectal metastasesin fatty liver using intraoperative ultrasound and histopathology as astandard of reference. Eur J Surg Oncol 38:670–676.26.
- Yu MH, Lee JM, Hur BY, Kim TY, Jeong SY, Yi NJ et al. (2015)Gadoxetic acid-enhanced MRI and diffusion-weighted imaging for thedetection of colorectal liver metastases after neoadjuvant chemotherapy.Eur Radiol 25:2428–2436.27.
- Cho JY, Lee YJ, Han HS, Yoon YS, Kim J, Choi Y et al. (2015) Role ofgadoxetic acid-enhanced magnetic resonance imaging in the preoperativeevaluation of small hepatic lesions in patients with colorectalcancer. World J Surg 39:1161–1166.28.
- Macera A, Lario C, Petracchini M, Gallo T, Regge D, Floriani I et al.(2013) Staging of colorectal liver metastases after preoperativechemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy.Eur Radiol 23:739–747.
Figures
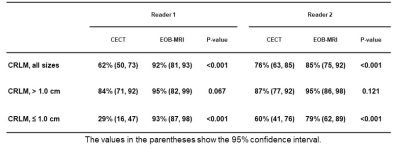
Sensitivity of gadoxetic
acid-enhanced MRI (EOB-MRI) versus contrast-enhanced computed tomography (CECT)
in detection of colorectal liver metastasis (CRLM).
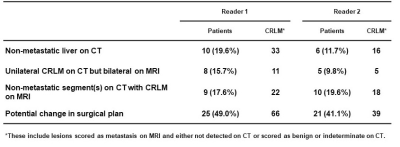
Patients with potential change in
surgical plan resulting from higher yield in detection of colorectal liver metastasis (CRLM) by EOB-MRI
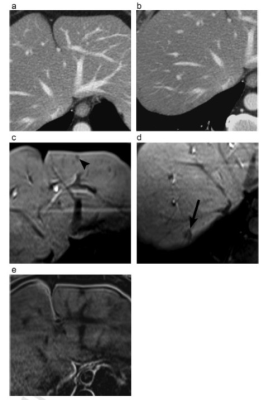
A 56-year-old man with
two histopathologically proven colorectal liver metastases (CRLM) seen on MRI
but invisible on CT. CT images (A and B) do not depict any lesion in the liver.
On hepatobiliary phase images of EOB-MRI (C and D) two CRLM are observed, in
segment 2 (arrowhead) and in segment 7 (arrow). Significant hepatic steatosis is
evident on subtraction MR image (E) obtained from chemical shift imaging.
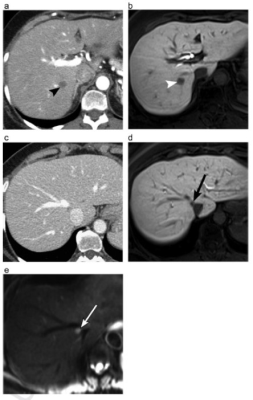
A 58-year-old
female with a metastasis (arrowheads) in segment 7 depicted on CT (A) and MRI
(B). While CT (C) shows no obvious metastasis in segment 8, an additional
definite metastasis (arrows) is seen in this segment on MRI, hepatobiliary
phase (D) and DWI (E), very close to the insertion of the middle hepatic vein
into IVC. This could impact the surgical plan compared to CT as now a more
extensive right hepatectomy and or middle hepatic vein reimplantation would be
necessary.