0069
Phase-encoded xSPEN: A novel high-resolution volumetric alternative to RARE MRI1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Department of Electrical Engineering and Computer Sciences, University of California, Berkeley, CA, United States
Synopsis
We have recently introduced cross-term SPatiotemporal ENcoding (xSPEN), a technique with exceptional resilience to field heterogeneities. This study explores a multi-scan extension of xSPEN, which simultaneously yields ky/kz data containing low and high frequency components as well as transposed low-resolution z/y images, with unique downsampling characteristics. A reconstruction scheme converting this information into high resolution 3D images with fully multiplexed volumetric coverage is introduced. The Results provide a series of high-resolution multiscan xSPEN imaging examples and analyzes their sensitivity vis-a-vis commonly used 2D RARE and multi-slab 3D RARE MRI techniques.
Purpose
To develop a rapid, non-CPMG high-resolution volumetric imaging approach, exhibiting a speed and in-plane resilience to field inhomogeneities comparable to RARE/turbo-spin-echo (TSE) while endowed with unique downsampling characteristics.Methods
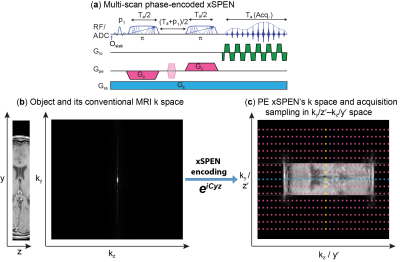
xSPEN is a single-shot imaging technique using frequency-swept pulses under the action of bipolar ±Gy and constant Gz gradients, to impart a hyperbolic phase eiCyz modulation; assuming a uniform object across the slice thickness Dz, subsequent readout by kz=gGzt provides what we referred to as a low-resolution y׳=kz/C profile.1 Resolution of this y-axis profile is given by the time-bandwidth product Q of the frequency-swept encoding pulses;1 here we seek to improve this resolution by performing additional ky encodings in independent scans (Fig. 1a).
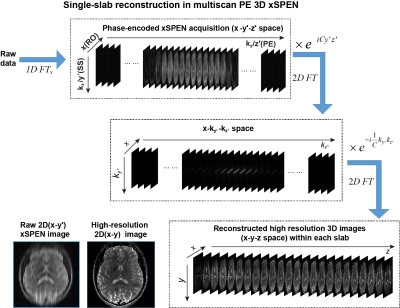
The bidimensional eiCyz kernel encoding spins in xSPEN leads to a unique situation, whereby the k-space sampling is spread from the well-localized situation of traditional MRI (Fig. 1b) into a 2D box-like shape (Fig. 1c). Single-shot xSPEN1 only captures the kz /y׳ axis for ky=0; conversely, should one collect signals under the action of Gy (kz=0), the outcome would be a ky/z׳ image. While this 2D kernel underlies xSPEN’s uniqueness, it is also the main source of SNR loss of these single-shot implementations. This study remedies this by introducing a ky-encoding that captures extended regions in k-space, improving SNR by Fourier multiplexing and resolution along the y-axis by probing higher kymax values. Furthermore, by exploring the z׳= ky/C profile, these ky-acquisitions lift the need to assume uniform slices, augmenting the z-axis resolution by a factor of Q. Taking into account that xSPEN’s EPI-like ±kx oscillations also yield a third, readout domain,2 suitable reconstruction of such data yields high in-plane resolution as well as ultrahigh though-plane resolution within the excited slab. Figure 2 summarizes one of the reconstruction procedures. Remarkable aspects of this new imaging approach are that (i) the k-space kernel provided by the hyperbolic phase allows sampling along ky to break the stringent Dky=1/FOVy criterion, (ii) enjoying full Fourier multiplexing, leading to high SNR and 3D high resolution images, (iii) restricted FOV imaging is a built-in ability without suffering from folding.
Results and Discussion
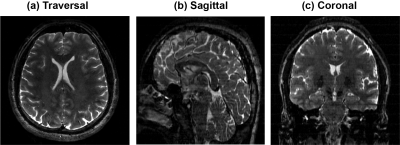
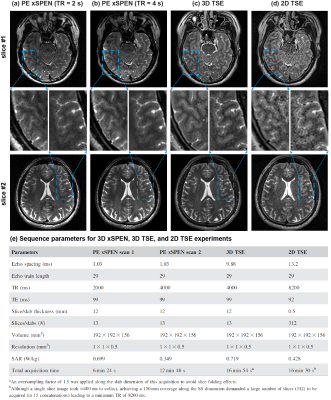
Experimental tests were carried out on human volunteers using Siemens 3T. Figure 3 illustrates high-resolution features of phase-encoded xSPEN MRI. These images arise from a PE xSPEN acquisition that covered the whole brain using an in-plane resolution of 1×1 mm2 and a resolution of 0.5 mm across the slab dimension. Figure 4 compare representative performances of multi-slab PE xSPEN acquisitions, against 3D multi-slab TSE and 2D multi-slice TSE results collected under as-close-as-possible acquisition conditions. Important criteria kept throughout these comparisons were equal FOV and resolution along all dimensions. The PE xSPEN method exceeded 2D TSE’s sensitivity by factors of ≈3-4, while providing similar resolution and SNR as 3D TSE in ≈50% acquisition times.
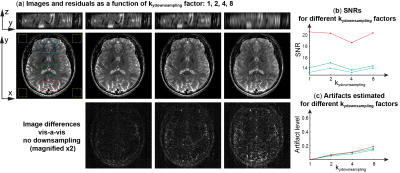
A remarkable aspect for multi-scan xSPEN MRI, was its ability to deal with a down-samplings of the ky phase-encoding variable. The folding that due to this down-sampling then occurs along the y axis, can be unfolded by the available low-resolution y′=kz/C profile –at the expense sacrificing resolution along the z axis and eventually increasing artifacts. Figure 5 demonstrates this feature by comparing fully ky sampled results, with images arising from down-sampled experiments. As can be appreciated from the y-z plane images only a substantial down-sampling of ky brings about strong effects in the resolution along z –which initially is mostly affected by a reduction in points. At first glance all these in-plane images look similar, and artificial structures are only visible when the down-sampling reaches Ry = 8. SNR estimations arising from different regions in the image also lack a clear trend as a function of down-sampling factors (Fig. 5b), even if artifact levels evaluated by taking differences against the images arising from a fully sampled experiment, do evidence a monotonic increase with down-sampling factors (Fig. 5c). The artifacts arising in these x/y images are dependent on the z-axis features for the various y-positions and show no particularly evident feature.
Conclusions
A multi-scan acquisition and reconstruction xSPEN strategy was introduced, capable of improving both the in-plane and through-plane resolutions. By relying on a Fourier processing full sensitivity is restored, while its special kernel permits a unique phase encoding acceleration. Further improvements including compressed sensing3, parallel imaging4 and multi-band excitation5 are also feasible. Furthermore, each phase encoding is a single-shot xSPEN acquisition providing a low-resolution 2D image; this may enable the scan-by-scan identification and elimination of motional artifacts, leading to very high definition 3D MRI capabilities.Acknowledgements
This work was funded by the Israel Science Foundation grant 795/13, ERC-2014-PoC grant # 633888, and the Kimmel Institute of Magnetic Resonance (Weizmann Institute). ZZ thanks Israel’s Council of Higher Education as well as the Koshland Foundation for postdoctoral fellowships. ML acknowledges a Visiting Faculty Program Fellowship (Weizmann). We are also grateful to Dr. Sagit Shushan (Wolfson Medical Center) and to the Weizmann MRI team (Edna Furman-Haran, Fanny Attar and Nachum Stern).References
1 Zhang Z., Seginer A., Frydman L., Single scan MRI with exceptional resilience to field heterogeneities. Magn Reson Med 2017;
2:623-634. 2 Mansfield P. Multi-planar image-formation using NMR spin echoes. J Phys C: Solid State Phys 1977;10:L55-L58.
3 Lustig M., Donoho, D., Pauly J., Sparse MRI: the application of compressed sensingfor rapid MR imaging, Magn Reson Med 2007; 58:1182-1195.
4 Barth, M., F. Breuer, P. J. Koopmans, D. G. Norris and B. A. Poser., Simultaneous multislice (SMS) imaging techniques. Magn Reson Med 2016;75: 63-81.
5 Griswold, M. A., P. M. Jakob, R. M. Heidemann, M. Nittka, V. Jellus, J. Wang, B. Kiefer and A. Haase. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magn Reson Med 2012;47: 1202-1210.
Figures