0068
Reverse double inversion-recovery: improving motion robustness of cardiac T2-weighted dark-blood turbo spin-echo sequence1Department of Radiology and Biomedical Imaging, Yale School of Medicine, New Haven, CT, United States, 2Department of Internal Medicine, Section of Cardiovascular Medicine, Yale School of Medicine, New Haven, CT, United States
Synopsis
The cardiac T2-weighted dark-blood turbo spin-echo (TSE) sequence based on double inversion-recovery (DIR) is subject to motion artifacts due to mismatching of slices from the dark-blood preparation and the TSE readout. Here we propose reverse double inversion-recovery (RDIR), which performs the slice-selective inversion of the DIR preparation in the same cardiac phase as the TSE readout to minimize the slice mismatching. RDIR was evaluated in healthy subjects and patients. Results show that RDIR-TSE achieved a significantly improved image quality in the right ventricle and an improved image quality in the left ventricle compared to the standard DIR-TSE.
Purpose
Cardiac double inversion-recovery turbo spin-echo (DIR-TSE) is commonly used to identify edema and visualize anatomy of the heart[1], with various advantages of strong myocardium-to-blood contrast, high spatial resolution, and robustness against field inhomogeneity due to high field or implanted devices,[2] compared to newer T2-mapping[3] or bright-blood T2-weighted methods[4]. However, the sequence is also well known to generate artifacts due to slice mis-registration between the DIR dark-blood preparation and the TSE readout, resulting in signal loss in the left ventricular (LV) and right ventricular (RV) myocardium and inconsistent image quality[4-7]. Here we propose and evaluate a novel dark-blood technique based on reverse double inversion-recovery (RDIR) to improve the motion robustness of the dark-blood TSE sequence.Methods
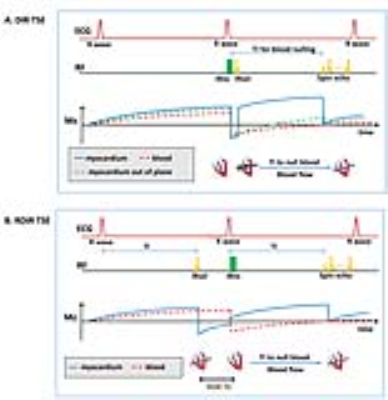
Figure 1 shows the difference between the DIR- and RDIR-TSE sequences and the impact of slice mis-registration. In DIR-TSE, the slice-selective inversion-recovery (IRsel) is performed after the nonselective inversion-recovery (IRns), both in begin-systole, while the TSE readout lies in the diastole. The cardiac phasic difference between IRsel and the TSE readout causes slice mis-registration, resulting in a signal loss in the affected myocardium (Figure 1A). A standard motion-compensation method is to double the IRsel slice-thickness[1]. In RDIR, the two inversion pulses are swapped in time, and IRsel is performed in the prior cardiac cycle at the same cardiac phase as the 90° RF pulse of the TSE readout. The phasic mismatching is thus eliminated and the risk of slice mis-registration is minimized (Figure 1B). Note that the rearrangement of IRsel does not increase the scan time since T2-weighted DIR-TSE acquires data in every second cardiac cycle.
RDIR-TSE was evaluated in 10 healthy subjects with a 3T scanner (Siemens Trio) and 20 patients with a 1.5T scanner (Siemens Avanto), with all subjects providing informed written consent. All imaging was performed in basal short-axis slice view due to its high mobility[6]. In healthy subjects, DIR- and RDIR-TSE were performed with matching parameters and two slice-thickness of IRsel, 200% and 110%, to test the performance of both sequences with or without slice-thickness compensation, respectively. Patients were imaged with a clinically used protocol and 200% IRsel slice-thickness for both DIR and RDIR. Images were assessed quantitatively, by measuring signal heterogeneity in the LV myocardium, signal heterogeneity in the RV myocardium, and the extent of RV signal dropout. The patient data was also assessed qualitatively by 2 radiologists blindly and independently with a 5-point scale.
Results
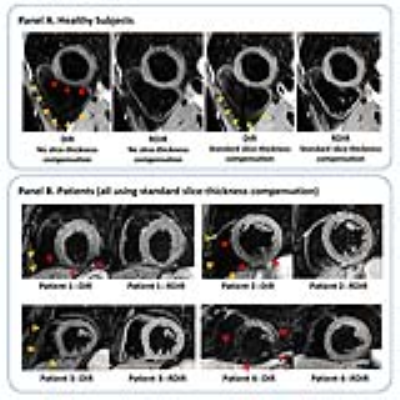
Figure 2 shows representative examples from a healthy volunteer (A) and four patients (B). Without slice-thickness compensation (IRsel slice-thickness: 110%), DIR-TSE led to an extensive dropout in the RV and heterogeneous signal in the LV, both of which were reduced with RDIR-TSE. With standard slice-thickness compensation (IRsel slice-thickness: 200%), there was still a dropout in the RV using DIR but not RDIR. In patients, while DIR led to clear dropouts in the inferior LV wall (subjects 1, 2, and 4) and RV wall (all 4 subjects), these artifacts were considerably reduced in their RDIR counterparts.
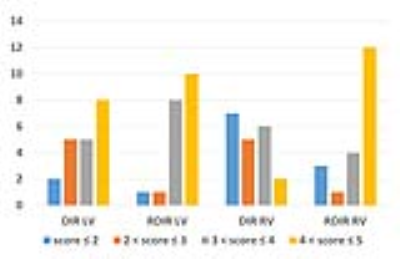
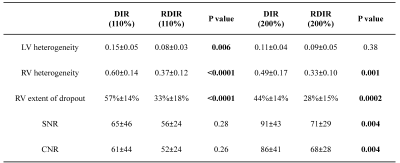
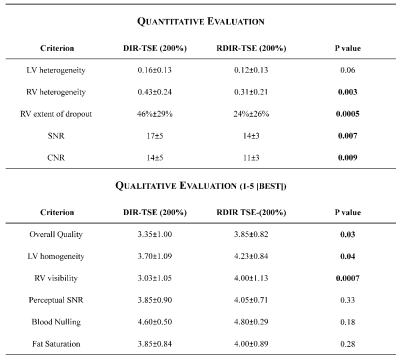
Tables 1 and 2 show the statistical comparison between DIR and RDIR over the healthy subjects and patients, respectively. In 10 healthy subjects (Table 1), RDIR led to a significant reduction of LV signal heterogeneity (p=0.006), RV signal heterogeneity (p<0.0001), and RV dropout (p<0.0001) without any slice compensation, and significant reduction of RV signal heterogeneity (p=0.001) and RV dropout (p=0.0002) with the standard slice compensation. In 20 patients (Table 2), the quantitative evaluation showed a significant reduction of RV heterogeneity (p=0.003) and extent of dropout (p=0.0005) with RDIR. The qualitative evaluation showed a significant improvement on overall quality (p=0.03), LV homogeneity (p=0.04), and RV visibility (p=0.0007) with RDIR. In Figure 3, the number of patients at each score was plotted against the score. RDIR increased the proportion of patients scored greater than 3 for both LV (90% vs 65%, p=0.13) and RV (80% vs 40%, p=0.02).
Discussion and Conclusions
The results show that RDIR-TSE achieved a significantly improved RV quality and an improved LV quality compared to the standard DIR-TSE. As both our data and previous literature[6] showed, slice-thickness compensation in DIR-TSE is less effective for highly mobile myocardial segments, such as basal inferior LV wall and RV wall. Alternative approaches, such as T2-mapping[3], improve LV imaging but may lack resolution for imaging of the RV or endocardial LV segments. RDIR-TSE may represent a valuable alternative for imaging of these structures in clinical assessment of edema and RV fatty infiltration. For the future, we plan to determine whether these improvements can impact the detection of edema in myocarditis.Acknowledgements
Financial support from grant NIH R01HL122560.References
1. Simonetti O, Finn J, White R: Black blood T2-weighted inversion-recovery MR imaging of the heart. Radiology 1996, 199.
2. Raphael CE, Vassiliou V, Alpendurada F, Prasad SK, Pennell DJ, Mohiaddin RH: Clinical value of cardiovascular magnetic resonance in patients with MR-conditional pacemakers. Eur Heart J Cardiovasc Imaging 2016, 17:1178-1185.
3. Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, Simonetti OP: T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson 2009, 11:56.
4. Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE: T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med 2007, 57:891-897.
5. Wince WB, Kim RJ: Molecular imaging: T2-weighted CMR of the area at risk—a risky business? Nature Reviews Cardiology 2010, 7:547-549.
6. Keegan J, Gatehouse PD, Prasad SK, Firmin DN: Improved turbo spin-echo imaging of the heart with motion-tracking. J Magn Reson Imaging 2006, 24:563-570.
7. Edelman RR, Botelho M, Pursnani A, Giri S, Koktzoglou I: Improved dark blood imaging of the heart using radial balanced steady-state free precession. J Cardiovasc Magn Reson 2016, 18:69.
Figures