0064
Novel Tumor-Selective Dual-Contrast 3D MRI Toward Zero False-Positiveness in Brain Metastases: A Feasibility Study1Center for Neuroscience Imaging Research, Institute for Basic Science (IBS), Suwon, Republic of Korea, 2Department of Brain and Cognitive Engineering, Korea University, Seoul, Republic of Korea, 3Department of Biomedical Engineering, Sungkyunkwan University (SKKU), Suwon, Republic of Korea
Synopsis
The purpose of this work is to develop a novel, tumor-selective dual-contrast 3D MRI technique that can clearly differentiate small brain metastases from contrast-enhanced vessels while potentially eliminating false-positiveness in the corresponding diagnosis. After injecting contrast agents, the proposed pulse sequence employs a pair of mixed encodings in each TR, yielding highly tumor-selective, blood-suppressed images from the latter to increase the sensitivity of metastases detection while producing blood-enhanced signals from the former to evaluate the false-positiveness of the detected metastases. It is expected that the proposed method enhances detection sensitivity to brain metastases while substantially reducing false-positiveness.
Purpose
The purpose of this work is to develop a novel, tumor-selective dual-contrast 3D MRI technique that can clearly differentiate small brain metastases from contrast-enhanced vessels while potentially eliminating false-positiveness in the corresponding diagnosis. After injecting contrast agents, the proposed pulse sequence employs a pair of mixed encodings (spoiled GRE and flow-sensitized FSE in succession) in each TR, yielding highly tumor-selective, blood-suppressed images from the latter to increase the sensitivity of metastases detection while producing blood-enhanced signals from the former to evaluate the false-positiveness of the detected metastases. It is expected that the proposed method enhances detection sensitivity to brain metastases while substantially reducing false-positiveness.Introduction
Contrast-enhanced T1-weighted 3D imaging [1] has been typically used to detect brain metastases by exploiting a T1-shortening effect of contrast agents penetrated into metastatic regions via loosened blood-brain-barrier. However, since the signal intensities in both metastases and blood vessels are similar, it is often difficult to differentiate the former from the latter. To tackle this problem, contrast-enhanced black blood MRI [2] was introduced, in which blood signals are nulled using flow-sensitized FSE while metastases signals are selectively enhanced. Nevertheless, in the presence of very slow blood flow in patients, blood signals may mimic metastatic lesions and thus result in false-positiveness. Given the above considerations, we propose to develop a novel, dual-contrast 3D MRI technique that yields simultaneous acquisition of blood-suppressed and blood-enhanced images in a single pulse sequence such that the former is used to detect metastatic regions while the latter is employed to evaluate false-positiveness. The feasibility of the proposed method toward zero false-positiveness is demonstrated in patient studies.Methods
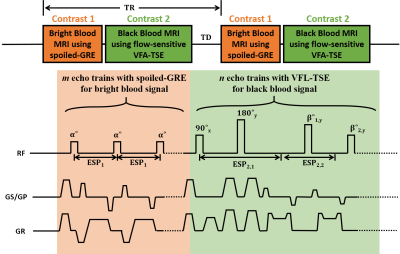
A schematic and timing diagram of the proposed, novel tumor-selective dual-contrast 3D MRI is shown in Figure 1. In each time of repetition (TR), a pair of mixed encodings with spoiled GRE and flow-sensitized FSE is applied in succession to acquire dual-contrast images. In the spoiled-GRE imaging module, bright blood signal is acquired with a centric reordering of phase encoding (out->in) to reduce signal modulation in the center of k-space. Additionally, the spoiled-GRE imaging module yields an inherent T1-preparation for the following FSE imaging module. At the end of the spoiled-GRE imaging module, a large crusher gradient is applied to remove remaining transverse magnetizations. Then, the flow-sensitized FSE module is applied, in which diffusion gradients are applied on the both sides of the first refocusing RF pulse to facilitate the attenuation of flow signals. To avoid loss of static signals due to the diffusion gradient induced non-CPMG condition, the flip angle of the first refocusing RF pulse is set to 180 degree. A centric reordering of phase encoding (in->out) is introduced to maintain T1-weighted contrast. Flip angles decrease rapidly from high to low values in the beginning of the echo train to establish pseudo steady state condition and then gradually increase up to 120 degree along the echo train [3]. Data were acquired in 5 patients on 3.0T (Siemens Verio, Seoul National University Hospital) using the proposed pulse sequence. Imaging parameters were: FOV, 256x194x160 mm3(sag); resolution 1x1x1mm3; TR, 650ms; total acquisition time, 8.6 min. The FSE imaging module was acquired with: TE, 19ms; turbo factor, 33; echo spacing (ESP2,2), 4.15ms. The spoiled-GRE imaging module was acquired with: flow compensation; echo spacing (ESP1), 7ms; flip angle, 10 degrees; number of average, 2. Images were then post-processed using MATLAB, FSL and Osirix Lite to: 1) extract metastatic signal regions by thresholding blood-suppressed images, 2) generate blood-enhanced vessel maps using maximum-intensity projection (MIP), and 3) combine metastatic regions with whole brain vessel maps to evaluate false-positiveness.Results and Discussion
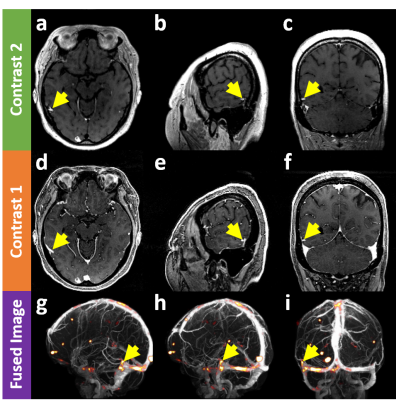
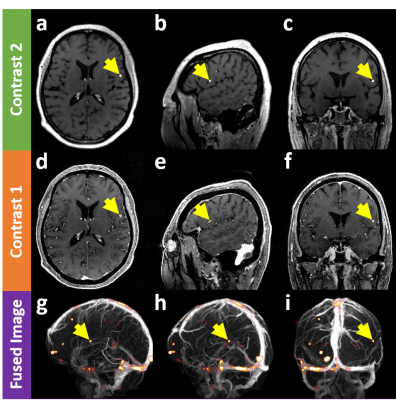
Figure 2 demonstrates that the proposed method can evaluate false-positiveness by detecting metastatic signals in the blood-suppressed images (a,b,c) and overlaying them on the vessel map (g,h,i) constructed using the blood-enhanced images (d,e,f). That is, if the metastatic signals of interest lie on the vessel map, there is little chance they turn out to be brain metastases (false positive signals). Figure 3 shows that the proposed method can detect brain metastases with high sensitivity while rejecting false positive signals. Unlike the case in Fig. 2, since the metastatic signals of interest in the blood-suppressed images (a,b,c) does not lie on the vessel map (g,h,i) constructed using the blood-enhanced image (d,e,f), there is a high probability that they turn out to be brain metastases.Conclusion
We successfully demonstrated the feasibility of the novel, tumor-selective dual contrast 3D MRI to enhance detection sensitivity in brain metastases while achieving nearly zero false-positiveness. It is expected that the entire evaluation process for diagnosis can be made automatic and clinical studies in a large population of patients will be then facilitated in the future.Acknowledgements
This research was supported by IBS-R015-D1 and the National Research Foundation of Korea (NRF) funded by the Ministry of Science (2016M3C7A1913844, NRF-2017R1A2B4012581).References
1. Mugler, John P., and James R. Brookeman. "Three‐dimensional magnetization‐prepared rapid gradient‐echo imaging (3D MP RAGE)." Magnetic Resonance in Medicine 15.1 (1990): 152-157.
2. Park, Jaeseok, and Eung Yeop Kim. "Contrast‐enhanced, three‐dimensional, whole‐brain, black‐blood imaging: Application to small brain metastases." Magnetic Resonance in Medicine 63.
3 (2010): 553-561. 3. Hennig, Juergen, Matthias Weigel, and Klaus Scheffler. "Multiecho sequences with variable refocusing flip angles: optimization of signal behavior using smooth transitions between pseudo steady states (TRAPS)." Magnetic resonance in medicine 49.3 (2003): 527-535.
Figures