0057
Simultaneous Multi-VENC and Multi-Slice (SMVMS) Phase Contrast Imaging Using Dual Steady-State Sequence1Helen Wills Neuroscience Institute, University of California, Berkeley, CA, United States, 2Advanced MRI Technologies, Sebastopol, CA, United States
Synopsis
Phase contrast (PC) MRI has been successfully applied to quantify flow velocity over the cardiac cycle by adding bipolar gradients with opposite polarity to cine gradient echo (GRE) sequences with prospective cardiac gating. However, PC-MRI suffers from a couple of disadvantages: 1) velocity aliasing which requires multiple acquisition without prior knowledge of the highest potential velocity and 2) excessive lengthy scan with whole brain coverage. In this work, we propose the Simultaneous Multi-VENC and Multi-Slice (SMVMS) technique to eliminate both issues. Instead of sequentially acquiring multiple slices with low and high velocity encoding (VENC) schemes, this interleaves an echo-shift technique in a Dual TR Steady-State (DSS) sequence using SMS excitation, which allows fast acquisition by sharing low- and high-VENC acquisitions with multiple slices in a single measurement.
Introduction
Phase contrast (PC) MRI has been successfully applied to quantify flow velocity over the cardiac cycle by adding bipolar gradients with opposite polarity to cine gradient echo (GRE) sequences with prospective cardiac gating1-2. However, PC-MRI suffers from a couple of disadvantages: 1) velocity aliasing may occur without prior knowledge of the highest velocity and require multiple acquisitions3 and 2) excessive lengthy scan time with whole brain coverage. In this work, we propose Simultaneous Multi-VENC and Multi-Slice (SMVMS) technique to eliminate both issues. Instead of sequentially acquiring multiple slices with low and high velocity encoding (VENC), this scheme interleaves an echo-shift technique in a Dual TR Steady-State (DSS) sequence using SMS excitation4, which allows faster acquisition by sharing low- and high-VENC acquisitions with multiple slices in a single measurement.
Methods
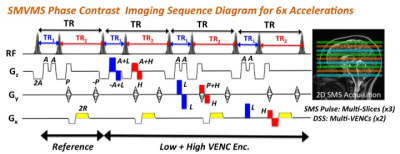
Bipolar gradients for VENC typically extend the echo time (TE) and repetition time (TR) especially in case of low-VENC gradient, thus prohibitively prolonging imaging time. Fig. 1 shows the SMVMS pulse sequence diagram with echo-shift gradient applied on the slice direction. In this sequence, low-VENC followed by an echo-shift gradient is just applied to the first TR (TR1) by eliminating ADC block with spatial encoding gradients to achieve minimum TR1 even in the presence of low-VENC gradient, while low- and high-VENC data are simultaneously acquired in the second TR (TR2), thus leading to DSS with different TRs to preserve imaging efficiency in a sequence. Furthermore, the use of SMS pulse provides an additional efficiency gain by simultaneously acquiring multiple slices as well as multiple VENCs in a single measurement, allowing desirable volumetric coverage.
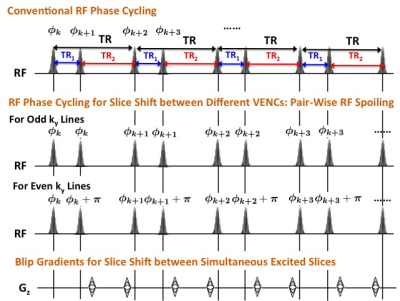
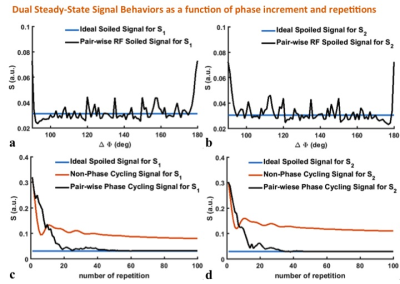
For slice separation along both VENC and slice directions, we employ a pair-wise RF spoiling for different VENCs and additional blip gradients for different slices, respectively, as shown in Fig. 2. To ensure complete spoiling of the transverse magnetization and investigate the influence of different TRs on potential steady-state artifacts, Fig. 3 shows the simulated steady-state signals as a function of RF spoiling phase increment ΔΦ (Figs. 3a and b) while the simulated transient of the SMVMS sequence signals to steady-states as a function of the number of TRs (Figs. 3c and d) with the following parameters: TR1/TR2=3/9 ms, T1/T2=1000/100 ms and flip angle (FA)=20°. It is observed that sufficient RF spoiling of both signals S1 and S2 is achieved simultaneously with a phase shift increment ΔΦ=130°.
Results
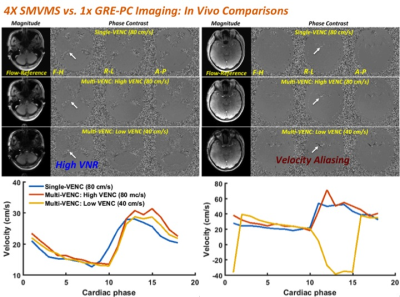
To demonstrate feasibility of the proposed SMVMS sequence in-vivo, a set of brain data was acquired in health volunteer with regular heart beats over all cardiac phases in sync with an external pulse oximeter. The imaging parameters were: TR1/TR2=3/9 ms, FA=14°, field-of-view (FOV) = 220x220mm2, slice thickness=5mm, matrix size=192x192, the number of cardiac phases=18, temporal resolution=42ms, SMS factor=2, low- and high-VENC=40/80cm/s. For slice separation, split-slice GRAPPA algorithm5 was applied offline using Matlab software (R2015b; Mathworks, Natick, MA). Figure 4 shows magnitude images from the flow-reference, the resulting PC images from three velocity directions, and the corresponding dynamic variation over the entire cardiac phases. Single-VENC acquisition typically makes the region of slow flow invisible in the presence of noises due to non-optimal VENC. Instead, SMVMS produces both high-VENC PC images while also capturing low velocity information with greater contrast owing to low-VENC gradient although it expectedly suffers aliasing of high velocity. The corresponding three velocity curves show good agreement over the entire cardiac phases as well as VENC aliasing consistent with qualitative PC images.
Discussions and Conclusions
This feasibility study demonstrates the potential utility of the simultaneous acquisition of multi-VENC and multi-slice using the novel DSS sequence with two different TRs. It was observed that the proposed acquisition scheme does not have image artifacts from steady-state issue while achieving slice shift between different VENCs. The proposed method can be further enhanced in imaging efficiency by either acquiring a faction of k-space in a Cartesian grid or manipulating spatial gradients on multiple axes for non-Cartesian acquisition. The proposed method is then expected to widen the clinical utility of the PC-MRI.
Acknowledgements
This research was supported by NIHR44MH11210.References
1. Firmin DN, Nayler GL, Klipstein RH, Underwood SR, Rees RSO, Longmore DB. In vivo validation of MR velocity imaging. J Comput Assist Tomogr 1987; 11:751-756.
2. Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR. Phase contrast cine magnetic resonance imaging. Magn Reson Q 1991; 7:229-254.
3. Lee AT, Pike GB, Pelc NJ, Three-point Phase contrast velocity measurements with increased velocity-to-noise ratio. Magn Reson Med 1995; 33:122-126.
4. Larkman DJ, Hajnal JV, Herlihy AH, Coutts GA, Young IR, Ehnholm G. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. J Magn Reson Imaging 2001; 13: 313-317.
5. Cauley SF, Polimeni JR, Bhat H, Wald LL, Setsompop K. Interslice leakage artifact reduction technique for simultaneous multislice acquisitions. Magn Reson Med 2014; 72:93-102
Figures