0056
High Spatial Resolution BOLD fMRI Using Simultaneous Multislice Excitation with Echo-Shifting Gradient Echo at 7 Tesla1Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2Department of Biomedical Engineering, Guangzhou Medical University, Guangzhou, China, 3Beijing MRI Center for Brain Research, Institute of Biophysics, Chinese Academy of Sciences, Beijing, China
Synopsis
Signal to noise gain at ultra-high field has pushed blood oxygen level dependent functional MRI towards high spatial resolution with the benefit of improved accuracy in functional mapping. However, the techniques available for high spatial resolution fMRI are mainly based on echo-planar imaging technique, which faces geometric distortion. In this work, we proposed a technique combining simultaneous multislice excitation with echo-shifting, which can be virtually free from distortion artifacts, for high spatial resolution fMRI. Significant activation was identified in visual and motor experiments with in-plane resolution of 1.0×1.0 mm2 and an acceleration factor of 10 at 7 Tesla.
Introduction
In neuroimaging, blood oxygen level dependent (BOLD) functional MRI (fMRI) is a main non-invasive method to map patterns of activation in the human brain. Conventional fMRI utilizes echo-planer imaging (EPI) to acquire high temporal resolution images. However, in high spatial resolution imaging at ultra-high field, EPI suffers from strong distortion artifacts because of its sensitivity to B0 field inhomogeneities. Therefore, EPI has difficulty in high spatial resolution fMRI at ultra-high field. To address this issue, we developed a novel sequence that combined simultaneous multislice excitation (SMS)1,2 with echo-shifting (ES)3 (named SMSiES) for high spatial resolution fMRI at ultra-high field.Theory and Methods
Sequence diagram was shown in Figure 1. For simplification, the diagram only illustrates two simultaneously excited slices with one echo shift factor. Additional gradients were implemented along both the slice and readout directions.
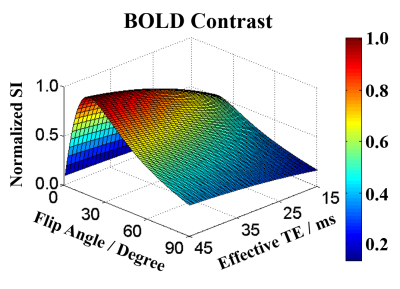
The Bloch simulation was conducted to optimize the flip angle and the effective TE for maximizing the BOLD contrast. The BOLD contrast CBOLD is defined as signal difference between the oxygenated and deoxygenated state of gray matter (GM), which can be given as: $$C_{BOLD}=M_{0}\cdot\sin\theta\cdot\frac{1-e^{-\frac{TR_{eff}}{T1}}}{1-\cos\theta\cdot e^{-\frac{TR_{eff}}{T1}}}\cdot\left(e^{-\frac{TE_{eff}}{T2^*_oxy}}-e^{-\frac{TE_{eff}}{T2^*_deoxy}}\right)$$ where M0 is the initial magnetization, θ is the flip angle and T1 is the T1 value of GM. TEeff is the effective TE and TReff is the effective TR. T2*oxy and T2*deoxy represent the T2* value of GM in oxygenated and deoxygenated state, respectively. The TReff was chosen as 27.6 ms, which equaled to that used in the in vivo experiments. The T2* values of GM in oxygenated and deoxygenated state were set to 27 ms and 25 ms4. The T1 value of GM was fixed as 2132 ms5. The flip angle varied from 0º to 90º with gap of 1º, and the TEeff ranged from 15 ms to 45 ms with increment of 1 ms.
All the in vivo experiments were performed on a 7.0 T MR system (MAGNETOM Trio, Siemens AG, Erlangen, Germany) with 32-channel head coil. IRB-approved 20 right-handed subjects with normal vision (9M, 11F, ages 24.4±3.1 years) were enrolled in this study. Two paradigms were designed to validate the feasibility of SMSiES in different fMRI applications. One utilized in visual task experiment used flash checkboard with the temporal frequency of 8 Hz. Starting from a tasking block, the visual paradigm consisted four 30-s tasking blocks showing flash checkboard, interspersed with four 30-s resting blocks. The motor task experiment requested the subjects to repeatedly make a fist with their left hand during tasking block. The motor paradigm started from a resting block, and consisted four 30-s tasking blocks interspersed with four 30-s resting blocks. The optimized flip angle of 9º was used in all fMRI experiments. Because of the limitation of sequence structure, an effective TE of 24.4 ms was utilized. Other parameters were: slices = 15, SMS factor = 5, ES factor = 2, GRAPPA acceleration factor = 2, voxel size = 1.0×1.0×2.5 mm3, acquisition time per volume = 3 s. The slice-GRAPPA and the in-plane GRAPPA algorithms were sequentially applied for image reconstruction6,7. SPM8 (from the Wellcome Dept. of Imaging Neuroscience, London) software package was used for fMRI data analysis.
Results
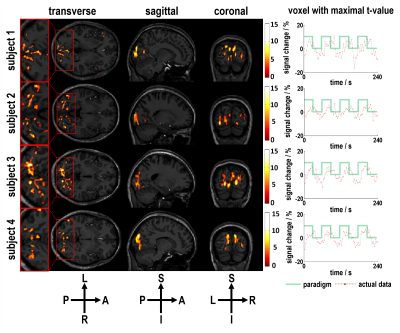
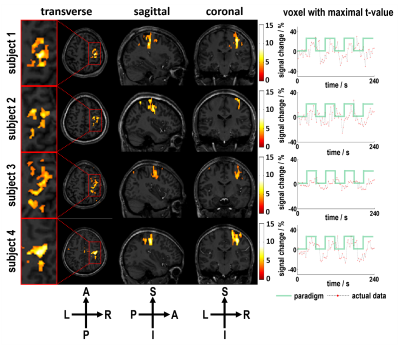
Simulations results showed that BOLD contrast reached its maximal value at the flip angle of 9º with the effective TE around 26 ms (Figure 2). Using the optimized imaging parameters, the feasibility of SMSiES in high spatial resolution fMRI was validated through visual (Figure 3) and motor (Figure 4) task experiments. Significant activation was identified in the bilateral visual cortex including calcarine inferior occipital gyrus with maximal t-value of 13.77 centered at [-33, -85, -12] (Figure 3, subject 2), and right primary motor cortex with maximal t-values of 8.76 centered at [33, -23, 48] (Figure 4, subject 4). Moreover, signal changes in both visual and motor experiments match well with the designed on-off paradigm (Figure 3&4).Discussion
High spatial resolution fMRI with in-plane resolution of 1.0×1.0 mm2 and an acceleration factor of 10 was realized using the proposed SMSiES sequence. Through the Bloch simulation, an optimized low flip angle 9º was utilized, resulting in a low power deposition even if the sequence conducted on 7 T with SMS factor of 5. Additionally, distortion-free BOLD images were obtained without any correction techniques. Moreover, both visual and motor task experiments demonstrated that the proposed sequence was able to effectively capture BOLD signal changes and manifest the potential for high spatial resolution fMRI at 7 T.Conclusion
SMSiES exhibits excellent BOLD sensitivity and can be virtually free from distortion artifacts. It could be used to detect BOLD signal originated from cortical areas near air-tissue interface. Therefore, SMSiES is a potential technique for high spatial resolution fMRI at ultra-high field.Acknowledgements
This work was supported in part by National Key Research and Development Program of China (2016YFC0100302); National Basic Research Program of China (2015CB755503); the National Natural Science Foundation of China (81571669, 61201442, 81401481, 61471350); the Natural Science Foundation of Shenzhen (GJHZ20150316143320494, KQCX2015033117354154, JCYJ20160429191938883, JCYJ20160331185933583, JCYJ20160531174850658); the Chinese National Major Scientific Equipment R&D Project (ZDYZ2010-2); the Natural Science Foundation of Guangdong (2017A050501026, 2014B030301013, 2014A030312006, 2015A020214019); the Strategic Priority Research Program Grants of Chinese Academy of Sciences (XDB02010001, XDB02050001) and the Excellent Youth Scholars of SIAT (Y5G003).References
1. Larkman D J, Hajnal J V, Herlihy A H, et al. Use of multicoil arrays for separation of signal from multiple slices simultaneously excited. Journal of Magnetic Resonance Imaging, 2001, 13(2): 313-317.
2. Breuer F A, Blaimer M, Heidemann R M, et al. Controlled aliasing in parallel imaging results in higher acceleration (CAIPIRINHA) for multi‐slice imaging. Magnetic resonance in medicine, 2005, 53(3): 684-691.
3. Ehses P, Bause J, Shajan G, et al. Efficient generation of T2*‐weighted contrast by interslice echo‐shifting for human functional and anatomical imaging at 9.4 Tesla. Magnetic resonance in medicine, 2015, 74(6): 1698-1704.
4. Donahue M J, Hoogduin H, van Zijl P, et al. Blood oxygenation level‐dependent (BOLD) total and extravascular signal changes and ΔR2* in human visual cortex at 1.5, 3.0 and 7.0 T. NMR in Biomedicine, 2011, 24(1): 25-34.
5. Rooney W D, Johnson G, Li X, et al. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magnetic Resonance in Medicine, 2007, 57(2): 308-318.
6. Setsompop K, Gagoski B A, Polimeni J R, et al. Blipped‐controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g‐factor penalty. Magnetic Resonance in Medicine, 2012, 67(5): 1210-1224.
7. Griswold M A, Jakob P M, Heidemann R M, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA). Magnetic resonance in medicine, 2002, 47(6): 1202-1210.
Figures