0055
Active, neuronal-activity-dependent trans-membrane water cycling detected by NMR1Interdisciplinary Institute of Neuroscience and Technology, Qiushi Academy For Advanced Studies, Zhejiang University, Hangzhou, China, 2Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States, 3Section on Critical Brain Dynamics, LSN, NIMH, National Institutes of Health, Bethesda, MD, United States, 4Section on Quantitative Imaging and Tissue Sciences, DIBGI, NICHD, National Institutes of Health, Bethesda, MD, United States
Synopsis
Transmembrane water cycling has long been thought an entirely passive, diffusion-dominated process. Recent studies suggest that an energetically active water cycling (AWC) mechanism, driven by Na+-K+-ATPase (NKA) pump activity, also occurs in different cell types, including neurons and astrocytes. Here we hypothesize that monitoring AWC could provide a new, more direct physical indicator of neuronal activity, which involves much ion cycling and enhanced NKA activity. By investigating neuronal populations in vitro under resting conditions with spontanotanous activity, and perturbed with tetrodotoxin (TTX), we found TTX simultanously reduces neuronal spiking activity and AWC (by >63%) suggesting AWC directly reflects neuronal activity.
Introduction
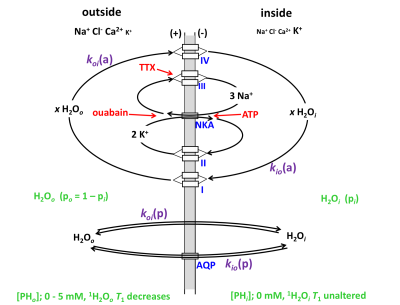
Water homeostasis and transport play important roles in brain function, e.g., ion homeostasis, neuronal excitability, cell volume regulation. However, specific mechanisms of water transport across cell membranes in neuronal tissue have not been completely elaborated. In addition to passive water cycling by water diffusing across cell plasma membranes, energetically active water cycling (AWC) also occurs in different cell types1,2, including neurons and astrocytes3,4. The AWC is largely driven by cell membrane Na+-K+-ATPase pump (NKA) activity, where water is co-transported with ion cycling across membrane using secondary active cotransporters to exit and enter the cell (Figure 1)2,5,6.
Neuronal activity is a highly metabolically demanding process that involves ion transport and recycling: NKA plays an essential role restoring ion gradients7. Recently, glutamate transporter activity was found to be strongly coupled to, and regulated by, NKA activity8. Here we hypothesize that AWC is postively correlated with neuronal activity, and monitoring AWC could provide a new, more direct physical indicator or measure of neuronal activity in vivo. To test this hypothesis, we investigated neuronal populations (organotypic cortical cultures (OCC) from newborn rat somatosensory cortex) under resting conditions in which spontanotanous activity is observed, and then treated with tetrodotoxin (TTX, a voltage-gated sodium channel (VGSC) blocker), to depress neuronal activity. We simultaneously measure the AWC and neuronal actitivity changes.
Methods
According to our hypothesis, the unidirectional rate constant for steady-state cellular water efflux, kio, has two components:
kio = kio(a) + kio(p) (1)
where a and p represent active and passive kio contributions, respectively. For passive exchange, kio(p) = <A/V>•PW(p), where <A/V> is the mean cell surface area/volume ratio, and PW(p) is the diffusion-driven passive membrane water permeability coefficient1,2. The active rate constant, kio(a), depends on the cellular metabolic rate of NKA, cMRNKA [e.g., pmol(ATP)/s/cell]. Thus, we rewrite kio as:
kio = {x/([H2Oi]•<V>)}cMRNKA + <A/V>•P (p) (2)
where [H2Oi] is the intracellular water concentration and x is the water stoichiometric multiplier (x = 500 to 1000 per co-transporter (I, IV in Fig. 1). In order to detect cMRNKA changes, kio(p) changes should be simultaneously estimated.
The kinetics of water exchange in OCC was measured using simultaneous, real-time fluorescence optical imaging and NMR. NMR signals were acquired during perfusion with a paramagnetic agent, Gadoteridol, which enables the determination of kio, and the mole fraction of intracellular water (pi), related to the average cell volume (V). Changes in intracellular calcium concentration, [Cai2+] were used as a proxy for neuronal activity and were monitored by fluorescence. Detailed experimental methods have been reported4.
Results
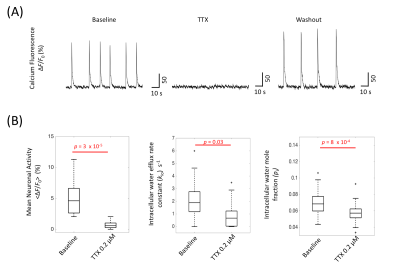
With normal artificial cerebrospinal fluid (ACSF) perfusion, the OCC exhibited spontaneous neuronal activity, demonstrated by the intracellular calcium fluorescence signal (Figure 2A), which exhibited brief (0.5 – 3 s) periods of neuronal excitation separated by ~10 s of very low activity9. This baseline spontaneous activity is largely reduced by adding 0.2 µM TTX (Fig. 2A). Interestingly, kio is also reduced, by 63%, from 2.05 (± 1.67) s-1 to 0.75 (± 1.13) s-1 (p = 0.03, Fig. 2B). The mean cell volume also was also reduced during TTX perfusion, with pi dropping from 0.070 (± 0.018) to 0.057 (± 0.015) (p = 8 x 10-4).
Assuming PW(p) and cell shape do not change, the kio(p) change from baseline to TTX can be estimated from Eq. 2:
{kio(p,TTX)/kio(p, baseline)} = {Vbaseline/VTTX}1/3 = 1/0.95 = 1.05 (3)
In this case, kio(p) increased by 5%, and, thus, kio(a) independently decreased by more than 63%.
Discussions and Conclusions
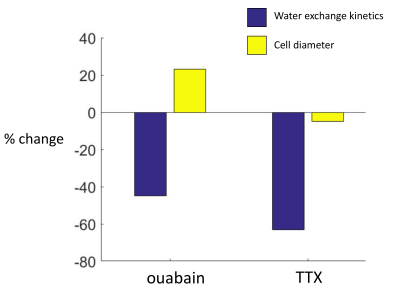
In this study, we found an AWC component in OCC, which is related to neuronal activity. With TTX perturbations, kio decreased by >63% while cell diameter (d) decreased by 5%. In a previous study with ouabain perturbation, kio decreased by 45% while d increased by a maximum of 23% (Figure 3)4. The two experiments demonstrate that d has no correlation with kio (Eq. 2), i.e., the active pathway must exist in neuronal tissue.
TTX is frequently used to modulate neuronal spiking activity by blocking VGSC, a Na+ influx channel. Here we show that TTX simultaneously reduces neuronal spiking activity and AWC (by at least 63%). A direct consequence of TTX binding would be to reduce Na+ influx, and thus decrease NKA activity, which pumps out Na+ to maintain the ion’s gradient, and to decrease the NKA activity-driven AWC component. It is probable that VGSC is one of the major III transporters shown in Fig. 1. Since its major activity is generally thought to occur during the action potential, our results suggest that kio, and specifically kio(a), directly reflects neuronal activity: cMRNKA increases during the spikes.
Acknowledgements
RB and PJB were supported by the Intramural Research Program (IRP) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH. RB was also supported by the 985 Program at Zhejiang University. We thank our colleague Craig Steward for helping prepare organotypic cultures. CSS acknowledges Drs. Craig Jahr, Thomas Barbara, Christopher Kroenke, Deborah Burstein, Robert Balaban, and Suzanne Scarlata for stimulating discussions, and the NIH [Grants UO1‑CA154602 and R44-CA180425] and the Paul G. Allen Family Foundation [Grant 12079] for his support. DP was supported by the Intramural Research Program (IRP) of the National Institute of Mental Health (ZIAMH002797), NIH.References
1. Zhang Y, Poirier-Quinot M, Springer CSS, Balschi JAA. Active trans-plasma membrane water cycling in yeast is revealed by NMR. Biophys J. 2011;101(11):2833-2842.
2. Springer CS, Li X, Tudorica LA, et al. Intratumor mapping of intracellular water lifetime: metabolic images of breast cancer? NMR Biomed. 2014;27:760-773.
3. Yang DM, Huettner JE, Bretthorst GL, Neil JJ, Garbow JR, Ackerman JJH. Intracellular water preexchange lifetime in neurons and astrocytes. Magn Reson Med. July 2017.
4. Bai R, Springer CS, Plenz D, Basser PJ. Fast, Na + /K + pump driven, steady-state transcytolemmal water exchange in neuronal tissue: A study of rat brain cortical cultures. Magn Reson Med. November 2017. Early View. doi:10.1002/mrm.26980).
5. Rooney WD, Li X, Sammi MK, Bourdette DN, Neuwelt EA, Springer CS. Mapping human brain capillary water lifetime: high-resolution metabolic neuroimaging. NMR Biomed. 2015;28(6):607-623.
6. Zeuthen T. Water-Transporting Proteins. J Membr Biol. 2010;234(2):57-73.
7. Reinhard L, Tidow H, Clausen MJ, Nissen P. Na+,K+-ATPase as a docking station: protein–protein complexes of the Na+,K+-ATPase. Cell Mol Life Sci. 2013;70(2):205-222.
8. Rose EM, Koo JCP, Antflick JE, Ahmed SM, Angers S, Hampson DR. Cellular/Molecular Glutamate Transporter Coupling to Na,K-ATPase. J Neurosci. 2009;29(25):8143-8155.
9. Bellay T, Klaus A, Seshadri S, Plenz D. Irregular spiking of pyramidal neurons organizes as scale-invariant neuronal avalanches in the awake state. Elife. 2015;4:e07224. .
Figures